|
Chemguide: Core Chemistry 14 - 16 The arrangement of electrons in atoms This page introduces the way that electrons are arranged in atoms. It looks in detail at the arrangement of the electrons in the first 20 elements in the Periodic Table in energy levels, and picks out useful patterns for some of the bigger atoms. Working out the number of electrons in an atom Remember that electrons carry a 1- charge, and protons carry a 1+ charge. In a neutral atom, there must be equal numbers of protons and electrons. The number of protons is the atomic number which you can find from a Periodic Table. So if, say, you are interested in sodium, Na, you will find that it has an atomic number of 11. That means that it has 11 protons, and the neutral atom must also have 11 electrons. Why do I keep stressing neutral atom? The number of protons in an atom defines what the atom is, and is therefore fixed for a particular element. But the number of electrons may vary. Sodium, for example, loses one of its 11 electrons when it forms compounds, but that changes the charge on the particle. It now has 11 protons but only 10 electrons - 11 positive charges, but only 10 negative ones. It would now be called a sodium ion, and given the symbol Na+. It is still a form of sodium because the number of protons hasn't change.
To form a negative ion, an atom would have to gain an extra electron (or more than one electron). For example, chlorine has an atomic number of 17, and the neutral atom must have 17 electrons. But chlorine commonly forms chloride ions, Cl-, by gaining an electron when it forms compounds with metals like sodium. In a chloride ion there will be 17 protons, but 18 electrons, and so it has a negative charge. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Note: When elements like chlorine or oxygen form compounds, their name changes to chloride or oxide. So we call the compound sodium chloride or sodium oxide, and not sodium chlorine or sodium oxygen. If you haven't done much chemistry yet, don't worry about this. It is something that you will just get used to without even thinking about it. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Introducing the arrangement of the electrons in an atom Let's start with the simplest atom, hydrogen, with 1 proton and 1 electron. The first thing you have to realise is that it is impossible to draw a diagram of this to scale. As a rough-and-ready calculation, if you drew the proton as a circle about 2 mm across, you would need a bit of paper about 200 metres across to fit the electron on it. Just be aware of this, but don't let it worry you! We just ignore the problem and draw atoms without any proper sense of scale - showing the electrons far closer to the nucleus than they really are. What is the electron in a hydrogen atom doing? A planetary model A model of the atom is just a way of thinking about it. It could be a real physical model, or a sketch on paper, or an idea in your head, or even a complicated mathematical equation. Popular science often shows electrons in atoms like planets orbiting around the sun - where the electrons are the planets and the nucleus is the sun. This is wrong! To plot a path for something you need to know exactly where the object is and be able to work out exactly where it's going to be an instant later. You can't do this for electrons for reasons you will meet if you do chemistry at a higher level. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Note: In order to plot a plane's course, it is no use knowing its exact location in mid-Atlantic if you don't know its direction or speed. Equally it's no use knowing that it is travelling at 500 mph due west if you have no idea whether it is near Iceland or the Azores at that particular moment. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
An energy level view of an electron So what is the electron doing in the atom? We don't know, we can't know, and so we just ignore the problem! What we do know is that a particular electron will have a particular definable energy. And that is the important point. When we draw electrons on circles around the nucleus (as we will shortly), those circles just refer to the energies of the electrons - the more distant the circle from the nucleus the higher the energy. You mustn't think that the electrons are moving around the nucleus along those circles. So the simplest atom, the hydrogen atom, would be shown as follows. The nucleus is usually represented by the symbol for the element and the electron can be shown as either a dot or a cross . . .
. . . and although in the hydrogen case it is common to draw the electron at the top, it doesn't really matter - you can put it wherever you need to.
The arrangement of the energy levels There are a whole lot more energy levels possible in an atom and, if you supply enough energy to an atom, the electron can jump around between them. But in the absence of any extra energy, the electrons will always be found in the lowest possible energy level - as close as possible to the nucleus. Each energy level can only hold a certain number of electrons, and the first level (closest to the nucleus) only has room for 2 electrons. The other levels we shall be concerned with hold increasing numbers of electrons.
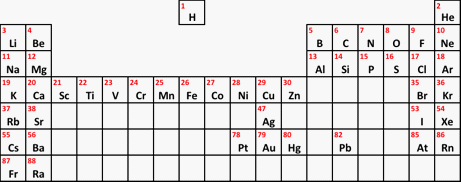
At this 14-16 year old level, we usually only look in detail at the arrangement of the electrons in the first 20 elements (up to calcium in the Periodic Table), and you probably won't need to worry about the fact that the third level can eventually take 18 electrons. The electron arrangements of the atoms from lithium to calcium in the Periodic Table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Note: You really need a full-sized paper copy of a Periodic Table which you can read easily. You will find a simple Periodic Table which you can download from this site. The download button is at the beginning of the second paragraph under the table. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Some important terms relating to the Periodic Table
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Important: There are a couple of problems here! The relatively unimportant one for the moment is that what I have called transition elements should more properly be called d-block elements. There is a subtle difference between the terms which you will come across if you do chemistry to a higher level. For now we will call them transition elements. The more important point concerns the way the groups are numbered. If you look at the copy of the Periodic Table I have suggested, you will find small numbers from 1 to 18 at the top of each group. This is the modern way of numbering the groups, but it has a big disadvantage if you are first learning chemistry. The old way of numbering the groups ignored the transition elements and numbered the other groups from 1 to 8 (or sometimes 1 to 7 and then 0 for the group headed by helium). As you will see further down this page, that is really useful if you are trying to work out electronic structures. The net effect is that for groups to the right of the transition elements, the old (and more useful!) number is 10 less than the modern one. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
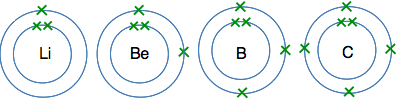
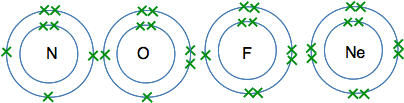
The atoms from lithium to neon In these atoms, the second energy level is gradually filling up with electrons. The second level holds 8 electrons, and that is why there are 8 atoms in this period. The atoms from lithium to carbon When we draw these, we normally show the electrons individually working around the circle.
The reason for showing the electrons like this is for convenience. It makes them easy to count, and single electrons are important in the way some atoms join together. The atoms from nitrogen to neon For these we normally draw the extra electrons paired with ones already there. That again makes is easier to count the electrons, and again is useful when we look at the way atoms bond to each other.
And at neon, the second level is full - there isn't room for any more electrons. The atoms from sodium to argon Now we start filling up the third energy level, and keep going until it becomes temporarily full with 8 electrons. I'm not going to draw these as circles with electrons on them, because the more electrons you have, the more of a bother it is to draw them all. Instead, we often use a quick short-hand to represent electronic structures. Sodium, for example, has 2 electrons in the first level, 8 in the second, and 1 in the third. We write this as Na 2,8,1 So the electronic structures in this period are
Potassium and calcium Because the third level is now temporarily full, the electrons in potassium and calcium have to go into the fourth level. Potassium is 2,8,8,1 and calcium is 2,8,8,2. Putting all the atoms from lithium to calcium together on one diagram
The blue numbers at the top of the table are the old group numbers. In a more modern table, the numbers from 3 to 8 would now be 13 to 18. As you will see in a moment, the older numbers (1 to 8) are much more useful. The really important electrons in any atom are always the outer ones. If you look at the numbers of outer electrons in any group, what do you find? In each group, the number of outer electrons in an atom is always the same, and is given by the (old) group number. That holds true for all the other atoms in each group, however complicated the rest of their electronic structure might be. So, for example, caesium towards the bottom of Group 1 has an electronic structure (which you wouldn't be able to work out at this level) of 2,8,18,18,8,1. Of all these electrons, the important one is the outer one. This is the one which is involved in all the chemistry of caesium. And you know that it has 1 outer electron because it is in Group 1. Similarly, although you couldn't at the moment work out the full electronic structure of iodine (towards the bottom of Group 7), you know that it must have 7 outer electrons. Why are hydrogen and helium missing from the table above? For different reasons, both of these are exceptions to what we have just looked at. Hydrogen is often written into the Periodic Table at the head of Group 1, above lithium. The Periodic Table I have suggested you look at does this - but it isn't somewhere that hydrogen fits properly. The key chemistry of Group 1 elements is that they are low melting point, very reactive metals. For example, they have a very vigorous reaction with cold water, getting more dramatic as you go down the group. Hydrogen is nothing like that! A useful compromise is to float hydrogen over the middle of the Periodic Table, suggesting that it is unique. That is what I did in the much simplified Periodic Table that you might have seen on another page.
Because the table of electronic structures above was squashed together, there was nowhere I could logically put the hydrogen without suggesting it was at the top of one of the other groups. I left helium out because is the only element in Group 8 (and it should indeed be in Group 8 or Group 0 as it is sometimes called) which doesn't have 8 electrons in its outer level. The first level can only hold 2 electrons. What is true of all the Group 8 elements is that their outer level is either full (He and Ne) or temporarily full with 8 electrons (the rest of the Group). What happens after these first 20 elements? You are very unlikely to need to know this in an introductory course at this level, but a quick look is interesting. After calcium the 3 level, which was temporarily full, continues to fill up completely. So for example: Scandium (Sc), the next element after calcium, has an electronic structure of 2,8,9,2. Iron (Fe), about half-way across the transition elements, is 2,8,14,2. Zinc at the end of the first row of transition elements, is 2,8,18,2. And then gallium (Ga) underneath aluminium in (old) Group 3 is 2,8,18,3. In other words, now we are in the right-hand block of the Periodic Table, we are back to the rule that the number of outer electrons is the same as the (old) group number. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Note: There are good reasons why the 3 level fills in two separate stages, but they are way beyond this introductory level. If you are really interested, you will find this discussed at a more advanced level in the main part of Chemguide. But don't even think about going there unless you are really confident about chemistry so far. If you are working towards an exam at this level, it would probably be better not to follow this up to avoid possible confusion. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
© Jim Clark 2019 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
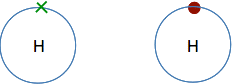
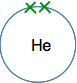 The next biggest atom is helium with two electrons. They are still in the same energy level, and would normally be drawn as a pair.
The next biggest atom is helium with two electrons. They are still in the same energy level, and would normally be drawn as a pair.