|
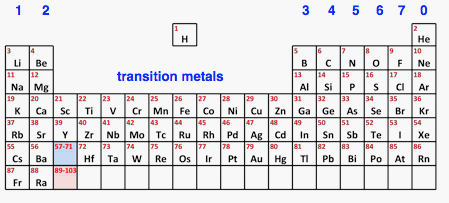
Chemguide: Core Chemistry 14 - 16 An Introduction to the Periodic Table This page introduces the important features of the Periodic Table. You may well have come across most of this earlier if you have been working through the site. Features of the Periodic Table Before you go on, you really need a full-sized paper copy of a Periodic Table which you can read easily. You will find a simple Periodic Table which you can download from this site. The download button is at the beginning of the second paragraph under the table. It contains much more information than you will need at this level. The order of the elements in the Periodic Table The elements are arranged in order of their atomic number. The atomic number counts the number of protons in the nucleus, and is sometimes more helpfully called the proton number. If you have downloaded the Table I suggested, the atomic numbers are the small numbers in green. Most Periodic Tables have two numbers against each element. The atomic number is always the smaller one. The bigger one is the relative atomic mass, and we aren't concerned with that at the moment. The simplified Periodic Table below just shows the atomic numbers.
Periods The horizontal rows are called periods. Notice that
The reason for the the various lengths has to do with the arrangement of electrons in energy levels. For example, the first level only holds 2 electrons (in hydrogen and helium). The second level fills up with another 8 electrons (from lithium to neon). The third level fills up with another 8 electrons (from sodium to argon). After that things get more complicated, and you don't need to know about it at this level. Groups Vertical columns are called groups. You will find a difference between the group numbering on most modern Periodic Tables (including the one you may have downloaded) and my version above. I am using the old version where what we think of as the main groups are numbered from 1 to 7 and then 0 for the far right one. You will also find the right-hand group numbered as Group 8. On this system of numbering, the columns in the transition metals were usually not numbered at school level. The big advantage of using the older method is that the group number tells you the number of electrons in the outer level of an element. (Group 0 is an exception to that. Helium has 2 outer electrons; the rest have 8.) If you know, for example, that caesium (Cs) is in Group 1, then you know that there is 1 electron in its outer level, however complicated the inner levels might be. If you know that bromine (Br) is in Group 7, then you know that there are 7 electrons in its outer level. Using the modern group numbers, where this group is numbered as Group 17, you can't do that. | |
|
Note: I have discussed this at more length on this page in the main part of Chemguide. Use the back button on your browser if you choose to follow this link. | |
|
Because the chemistry of an element depends on the outer electrons, you would expect elements in the same group to have similar properties. That is true for the groups that we will look at (Groups 1, 7 and 0) - their chemistry is very similar although there are trends within that similarity. For example, Group 1 contains very reactive metals, but their reactivity increases as you go down the group. In the groups in the middle of the table, the differences between top and bottom of the group can be quite large, but that isn't a problem that will concern you unless you do chemistry at a higher level. The position of hydrogen in the Periodic Table Many Periodic Tables (including the one I suggested you use) place hydrogen at the top of Group 1. That's not ideal! Hydrogen is a gas; Group 1 elements are very reactive metals. Hydrogen doesn't fit easily in that spot. Actually it doesn't fit easily at the top of any group. The best compromise is just to accept that it is different and float it somewhere in the middle - as I have done with my version of the Table. Transition metals The transition metals contain the elements that you think of as being typical metals - copper, iron, chromium, titanium and so on. We will have a brief look at some of their characteristic properties on another page in this section. | |
|
Note: There are, of course, common metals elsewhere in the Periodic Table as well - magnesium, aluminium and lead, for example. | |
© Jim Clark 2020 |
|