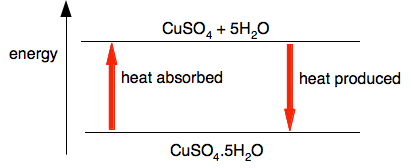|
Chemguide: Core Chemistry 14 - 16 An introduction to reversible reactions and chemical equilibria This page starts with a couple of simple reversible reactions, and then extends it to what happens if such a reaction is in a closed system - introducing the important idea of a dynamic equilibrium. A more detailed discussion of how you can alter such an equilibrium by changing the conditions will be found on separate pages in this section. You might need to refer to the page about energy changes in reactions if you aren't happy with terms like "exothermic", "endothermic" and "energy diagram". Two simple reversible reactions The effect of heat on copper(II) sulfate crystals You are probably familiar with the use of anhydrous copper(II) sulfate as a test for water - if not it doesn't matter. Blue copper(II) sulfate crystals contain water of crystallisation - water which is chemical bound into the crystal. The formula is CuSO4.5H2O. When it is heated, this happens:
This reaction doesn't work if you don't heat it - it is an endothermic change. The video shows this happening: It also shows that the reaction can be reversed by adding water back to the white anhydrous copper(II) sulfate.
What it doesn't show is that the reverse reaction is strongly exothermic - the mixture after you have added the water gets very hot. If you look at this reaction on an energy diagram, that isn't very surprising.
However much heat you put in to split the copper(II) sulfate from the water, you are bound to get exactly the same amount produced when the reaction reverses. You can show this pair of reactions using reversible arrows.
The effect of heat on ammonium chloride The photo shows what has happened after some white ammonium chloride powder has been heated strongly for a time.
The white solid collected further up the tube is also ammonium chloride. What happens is that the ammonium chloride breaks up on heating into colourless ammonia and hydrogen chloride gases. These travel up the tube until it gets cool enough for them to recombine into ammonium chloride. The reversible reaction is :
The forward reaction (the reaction in the equation from left to right) is endothermic. The back reaction (the reaction in the equation going from right to left) is exothermic. This bit of video usefully summarises what we have talked about so far, and makes a nice introduction to what comes next. Reversible reactions in a closed system: dynamic equilibrium A closed system is one in which no substances can be removed or added. On the other hand, heat can transfer in or out. In the examples above, heating copper(II) sulfate is being carried out in an open system - the water being formed escapes as steam. The ammonium chloride is also being heated in an open test tube. Although most of the ammonia and HCl recombine further up the tube, some will be lost from the top. In fact ammonia molecules travel faster than HCl molecules and escape from the top of the tube before the HCl. So the system isn't properly closed. The best examples of reversible reactions in a closed system involve chemistry that you probably won't have come across yet. So I just want to generalise by considering this reversible change:
A, B, C and D can be all in the liquid state or all gases for simplicity. Suppose you start with A and B. At the beginning, there will be maximum amounts of A and B, and so the rate of the forward reaction will also be at its maximum. As the reaction continues, the amounts of A and B will reduce, and so the rate reduces as well. Now consider C and D. Initially, there isn't any C and D, and so the rate of the back reaction will be zero. But as more and more C and D get formed, the rate of the back reaction will speed up. If the system is closed, there will come a point when the two rates become the same. Every A and B which gets turned into C and D will be replaced by the same amount produced by the back reaction. And every C and D which gets used up will in turn be replaced by the same amount produced by the forward reaction. As fast as something is being removed, it is being replaced again by the reverse reaction. When that happens, there will be no further change in the amounts of anything in the reaction. We call this a state of dynamic equilibrium. "Dynamic" because the reaction is still going on; "equilibrium" because of the lack of change in the amounts of everything. Position of equilibrium Depending on the conditions and the exact nature of the reaction, the equilibrium mixture could end up with mostly A and B, similar amounts of everything, or mostly C and D. It depends on which of the forward or back reactions happens more readily. Here is the equilibrium again.
If the equilibrium mixture has a majority of A and B, we say that the position of equilibrium lies to the left, or lies towards A and B. If the equilibrium mixture has a majority of C and D, we say that the position of equilibrium lies to the right, or lies towards C and D. As you will see later on, the position of equilibrium can be changed by changing the conditions of the reaction. That is important because you are rarely interested in the equilibrium mixture. You do a reaction in order to make one of the products - you need to move the position of equilibrium as far to the right as you can. Changing the conditions to move the position of equilibrium - Le Chatelier's Principle Le Chatelier's Principle says:
It is important to realise that Le Chatelier's Principle is no more than a useful guide to help you work out what happens when you change the conditions in a reaction in dynamic equilibrium. It doesn't explain anything. It is very important that you check your syllabus to find out whether Le Chatelier's Principle is mentioned. If it is, you can quote it in an exam. If it isn't, do not under any circumstances quote it in an exam. That doesn't mean that you can't use it to help you to work out what is going to happen if you change something, but some examiners may well penalise you for using the words "Le Chatelier's Principle". If that is the case, I will always give you a way of phrasing it that will get around that problem. We are going to look at the effect of changing conditions on the position of equilibrium over a number of pages. I am introducing it now so that I only have to write this warning once.
© Jim Clark 2020 |