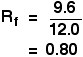|
Chemguide: Core Chemistry 14 - 16 Paper chromatography This page introduces chromatography as a way of separating small amounts of mixtures. It only deals with the simplest version of chromatography using coloured substances and paper. How does paper chromatography work? There are two simple videos (from the same source that you may have already met in distillation). The first one introduces some important words and ideas. The second one takes this further and looks at the sort of practical that UK examiners may well expect you to have done at this level. In a real experiment, you would also have the colours of the spots to help you decide which the components of the mixture were. But colour isn't enough - the Rf values are critical. A bit more about Rf values Some compounds in a mixture travel almost as far as the solvent does; some stay much closer to the base line. The distance travelled relative to the solvent is a constant for a particular compound as long as you keep everything else constant - the type of paper and the exact composition of the solvent, for example. The distance travelled relative to the solvent is called the Rf value. For each compound it can be worked out using the formula:
For example, if one component of a mixture travelled 9.6 cm from the base line while the solvent had travelled 12.0 cm, then the Rf value for that component is:
In the example on the second video, it probably wasn't necessary to measure Rf values because you are making a direct comparison just by looking at the chromatogram. You are making the assumption that if you have two spots in the final chromatogram which are the same colour and have travelled the same distance up the paper, they are most likely the same compound. It isn't necessarily true of course - you could have two similarly coloured compounds with very similar Rf values. You could check that possibility by using a different solvent. Rf values only apply to a particular paper and a particular solvent. This isn't something you will have to worry about at this level. Finally . . . At the time of writing, one UK GCSE syllabus also mentioned the use of thin layer chromatography. This works in exactly the same way as paper chromatography, but uses a thin layer of solid coated onto a small glass plate rather than paper. If you need this, you will find a page about thin layer chromatography by following this link to the advanced part of the site. You probably won't need to know about the section on how thin layer chromatography works. If you follow up on this, use the BACK button on your browser to get back here. It is in a fairly distant part of the site!
© Jim Clark 2021 |