|
Chemguide: Core Chemistry 14 - 16 Filtration and crystallisation This page introduces some key words, and looks at how you can separate a mixture of a solution and a solid. Some key terms to start with
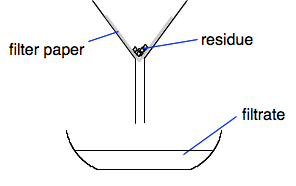
Be very careful with the use of the terms "clear" and "colourless". A colourless liquid is one which has no colour. A clear liquid is one which you can see through, but can be any colour. Separating a mixture of a soluble and insoluble substance Suppose you had several small diamonds which you wanted to protect from being stolen. You come up with the idea of mixing them with salt crystals of the same sort of size, and putting them in a jar in your store cupboard labelled "SALT". But now you want your diamonds back again. Making a solution If you stirred the mixture with an excess of water, the salt would dissolve, but the diamonds won't. So you filter the mixture by pouring it through filter paper in a funnel. The diamonds would remain on the filter paper, and the salt solution would pass through. More key terms
So in this case, the salt solution is the filtrate, and the diamonds are the residue.
Cleaning up the residue At the moment, the diamonds are coated with salt solution. Even if you let them dry, they would still be coated in salt. So the residue is washed on the filter paper with several small amounts of pure water. Finally, you can just let them dry. In summary, to get a pure solid from a mixture of a solid and a solution:
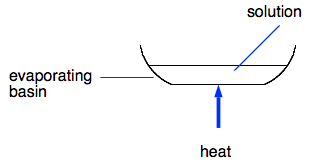
Recovering the dissolved solid from the solution We will just look at this in general terms, because it is pretty unlikely that you would want to recover a small amount of salt. The process is called crystallisation. The filtrate is heated gently in an evaporating basin.
In most cases, you can't simply evaporate the solution to dryness. A lot of the substances that you might want to make pure in this way have crystals which have water as an essential part of their crystals. This is called water of crystallisation. So you normally only evaporate off enough water so that the solid will form crystals when the solution cools. The video shows all of this process (filtration and evaporation) used to make pure copper(II) sulfate crystals from copper(II) oxide and dilute sulfuric acid. You will meet this again later in the course. There is one slight oddity about this video in the way he demonstrates folding a filter paper. He over-folds it in the demo, but when he fits it into the filter funnel, it is folded properly! In case you missed it, the thermometer reads about 60°C. How do you know when enough water has been evaporated off? The simplest way is to take a very small sample, cool it, and see if it crystallises. You can do this by dipping a glass rod into the solution as you are heating it and picking up a drop of liquid on the end. If you wave this around gently in the air, you can see whether or not crystals will form when the solution cools. Then you allow the whole solution to cool so that crystals form. If you leave it for a day or so, all the water usually evaporates off to leave just dry crystals. If you want to short-cut this, you can pour off any uncrystallised solution and dry the crystals on filter paper.
© Jim Clark 2021 |