|
Chemguide: Core Chemistry 14 - 16 States of Matter: Solids, liquids and gases This page takes an introductory look at the way particles are arranged in solids, liquids and gases, and the attractive forces which exist between them. Its purpose is really just to get you thinking about things in terms of particles. A particle view of matter 
The photo shows a glass of water with a stainless steel spoon on a wooden table, and has examples of all the states of matter. There are three solids - the metal spoon, the glass and the wooden table. There is liquid water. And, of course, there is air all around it - a mixture of gases. Let's start by looking at the arrangement of particles in solids. Solids All of the solids in the photo have a fixed shape. The glass can't penetrate the wood; the metal spoon doesn't penetrate the glass. That means that the particles that make them up must be held tightly and strongly together with not much space between them. 
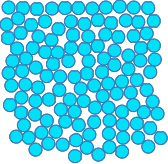
The typical picture of the arrangement of particles in a solid that you will find in most sources at this level looks like this. The particles are packed closely together in a regular way with strong forces of attraction between them. The particles obviously can't move around, but they do vibrate. At any temperature above absolute zero, there will always be movement of some sort. If you are asked to describe the arrangement of particles in a solid at this level, that is probably what your examiners are looking for. But, as with all exams, you must know exactly what your particular examiners want. Download a copy of your syllabus (specification) and find past papers and mark schemes and any other useful information available from your Exam Board's website. If you are doing a UK-based syllabus, you can find links to the Exam Boards' websites where you can download a copy of your syllabus and other useful stuff on the about this part of Chemguide page. Now stop and think! If you stop and think about this, the simple model described above can't be literally true! Why not? Suppose you had a hammer and nail, and attacked the metal spoon with it. You might possibly scratch or dent it, but nothing much else will happen. The model above is consistent with that. But suppose you did the same thing with the wooden table. The nail would force the wood apart and you could drive the nail deep into the wood. Then think about what unpolished natural wood looks like. It has grain - it isn't the same in all directions. None of that is consistent with the simple model. Now imagine attacking the glass with the nail and hammer. It will just shatter. The simple model can't account for that either. The solid in the photo which is closest to the diagram above is the stainless steel, but stainless steel is actually a mixture (an alloy) of iron, chromium and possibly nickel and other elements as well. That means that it will contain various different sized particles and the packing of the spheres will be more complicated. Wood is a complex mixture of organic compounds which are certainly nothing like round! And glass is complicated - it doesn't have a regular packing structure at all. | |
|
If you are interested: There is a long Wikipedia article on wood which you might like to look at, and you might also have a quick look at this article showing the structure of glass. Nobody would expect you to remember any of this! | |
|
So what exactly do you need to remember? Solids consist of closely packed vibrating particles with strong enough forces between them to hold the whole thing together. We usually simply draw the particles as being round just because it is easy. Not all solids have regular packing. Liquids
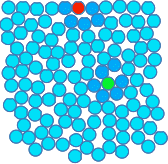
This diagram of the arrangement of particles in a liquid has the same number of particles as in the diagram of the solid, but it takes up a bit more room.
Notice:
Why is the top surface of an undisturbed liquid flat? This is a repeat of the liquid diagram above, but I have painted two of the particles red and green. I have also slightly darkened the other particles surrounding the red and green ones. None of these particles are different from before - I just want to be able to pick them out to talk about them.
Look first at the green particle - a particle in the middle of the liquid. There are attractions between it and all of the particles surrounding it. The closer the particles are together, the stronger the attraction. This green particle has other particles above and below it, to the left and right of it, and in front of it and behind it. It is being attracted in all directions. On average, all those attractions will cancel out, and the green particle isn't going to be pulled in any particular direction over time. Compare that with the red particle in the surface of the liquid. It is being attracted to particles to either side of it - on average, they will cancel out. It is being attracted by particles in front of it and behind it - on average, they will also cancel out. But there aren't any liquid particles above it, and there will be a permanent net attraction downwards into the liquid by the particles underneath it. Since this is happening to all the particles in the surface layer, you will get a flat top surface. If a lump formed on the surface, then the attraction of the particles underneath the lump will just flatten it out again. But sometimes the top surface of an undisturbed liquid isn't flat! 
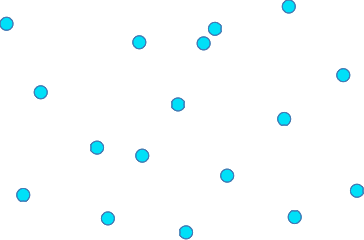
At the edge of a liquid, where it touches its container, the liquid surface may curve. This photo from the US Geological Survey site shows water in a burette. You can see that the water surface curves upwards where it meets the glass. The darkening around the top is just an effect of the light. This curved surface is known as a meniscus. So why does a water surface curve up when it meets the glass? At this point there are forces between particles in the glass and the water particles as well as between the water particles themselves. Now, the attractions of the water particles to the glass are greater than those between themselves. The downward forces on the surface water molecules aren't strong enough to overcome the forces attracting the water to the glass. What if you had a liquid where the forces between the particles in the liquid were stronger than between the liquid and the glass? A good example is liquid mercury in a glass tube. There are quite strong forces between the mercury particles, but only very weak ones between the mercury and the glass. In this case the meniscus curves the other way. I can't find a good clear copyright-free photo of this, but you will find a simple diagram if you follow the link to the US Geological Survey site mentioned above. Gases Gases are very straightforward! The particles are free to move in all directions, and as a result the gas will fill whatever you put it in. As a reasonable approximation, on average the particles in a gas at ordinary pressures are spaced about 10 particle diameters apart. Don't take that too literally - it obviously depends on how big the particles are. But when you draw the particles in a gas, leave a lot of space between them.
The particles are moving so fast, and the attractions between them are so weak, that if they collide they don't stick together - they just bounce apart. it is essential that you don't imagine that all the particles are moving at the same speed. There will be a huge range of speeds because after each collision one particle is likely to speed up and the other slow down - that is happening all the time. This will turn out to be very important when you look at factors which affect the rates at which reactions happen.
© Jim Clark 2018 |
|