|
Chemguide: Core Chemistry 14 - 16 Giant metallic structures This page describes the way the arrangement and bonding of metal atoms in a giant metallic lattice leads to the simple physical properties of metals I am assuming that you already know about metallic bonding. The current page builds on that page, and so it is essential that you have read it recently. You will find this page goes into a bit more detail than is usual at this level. That's because I think it is interesting, and not very difficult. The arrangement of the atoms Metals are giant structures of atoms held together by metallic bonds. "Giant" implies that large but variable numbers of atoms are involved - depending on the size of the bit of metal. Most metals are close packed - that is, they fit as many atoms as possible into the available volume. Each atom in the structure has 12 touching neighbours. Such a metal is described as 12-co-ordinated. Some metals (notably those in Group 1 of the Periodic Table) are packed less efficiently, having only 8 touching neighbours. These are 8-co-ordinated. Crystal grains It would be misleading to suppose that all the atoms in a piece of metal are arranged in a regular way. Any piece of metal is made up of a large number of "crystal grains", which are regions of regularity. At the grain boundaries atoms have become misaligned.
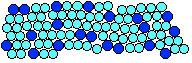
You can actually see these individual crystal grains on a newish piece of galvanised iron. Galvanised iron is iron coated with zinc, and the shiny patterns on the surface are crystal grains in the zinc.
| |
|
Note: This photo comes from Wikipedia. | |
|
The physical properties of metals Melting points and boiling points Metals tend to have high melting and boiling points because of the strength of the metallic bond. The strength of the bond varies from metal to metal and depends on the number of electrons which each atom delocalises into the sea of electrons, and on the packing. Group 1 metals like sodium and potassium have relatively low melting and boiling points mainly because each atom only has one electron to contribute to the bond - but there are other problems as well:
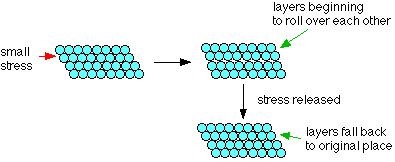
But even a low melting point metal like sodium (melting point 98°C) has a much higher melting point than the atom before it in the Periodic Table, neon (melting point -249°C). Electrical conductivity Metals conduct electricity. The delocalised electrons are free to move throughout the structure in 3-dimensions. They can cross grain boundaries. Even though the pattern may be disrupted at the boundary, as long as atoms are touching each other, the metallic bond is still present. Liquid metals also conduct electricity, showing that although the metal atoms may be free to move, the delocalisation remains in force until the metal boils. Thermal conductivity Metals are good conductors of heat. Heat energy is picked up by the electrons as additional kinetic energy (it makes them move faster). The energy is transferred throughout the rest of the metal by the moving electrons. Strength and workability Malleability and ductility Metals are described as malleable (can be beaten into sheets) and ductile (can be pulled out into wires). This is because of the ability of the atoms to roll over each other into new positions without breaking the metallic bond. If a small stress is put onto the metal, the layers of atoms will start to roll over each other. If the stress is released again, they will fall back to their original positions. Under these circumstances, the metal is said to be elastic.
If a larger stress is put on, the atoms roll over each other into a new position, and the metal is permanently changed.
The hardness of metals This rolling of layers of atoms over each other is hindered by grain boundaries because the rows of atoms don't line up properly. It follows that the more grain boundaries there are (the smaller the individual crystal grains), the harder the metal becomes. Offsetting this, because the grain boundaries are areas where the atoms aren't in such good contact with each other, metals tend to fracture at grain boundaries. Increasing the number of grain boundaries not only makes the metal harder, but also makes it more brittle. Controlling the size of the crystal grains If you have a pure piece of metal, you can control the size of the grains by heat treatment or by working the metal. Heating a metal tends to shake the atoms into a more regular arrangement - decreasing the number of grain boundaries, and so making the metal softer. Banging the metal around when it is cold tends to produce lots of small grains. Cold working therefore makes a metal harder. To restore its workability, you would need to reheat it. You can also break up the regular arrangement of the atoms by inserting atoms of a slightly different size into the structure. Alloys such as brass (a mixture of copper and zinc) are harder than the original metals because the irregularity in the structure helps to stop rows of atoms from slipping over each other.
© Jim Clark 2019 |
|