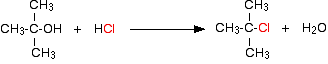|
REPLACING THE -OH GROUP IN ALCOHOLS BY A HALOGEN This page looks at reactions in which the -OH group in an alcohol is replaced by a halogen such as chlorine or bromine. It includes a simple test for an -OH group using phosphorus(V) chloride. Reactions involving hydrogen halides The general reaction looks like this:
Reaction with hydrogen chloride Tertiary alcohols react reasonably rapidly with concentrated hydrochloric acid, but for primary or secondary alcohols the reaction rates are too slow for the reaction to be of much importance. A tertiary alcohol reacts if it is shaken with concentrated hydrochloric acid at room temperature. A tertiary halogenoalkane (haloalkane or alkyl halide) is formed
| ||
|
Note: If you don't know what primary, secondary and tertiary alcohols are, you should read the introduction to alcohols before you go on. The terms primary, secondary and tertiary are used in exactly the same way with halogenoalkanes. Use the BACK button on your browser to return to this page. | ||
|
Replacing -OH by bromine Rather than using hydrobromic acid, you usually treat the alcohol with a mixture of sodium or potassium bromide and concentrated sulphuric acid. This produces hydrogen bromide which reacts with the alcohol. The mixture is warmed to distil off the bromoalkane.
| ||
|
Note: You will find practical details of this reaction on the page about preparation of halogenoalkanes. You don't need to read the beginning of that page because it is just a modification of this one! Use the BACK button on your browser to return to this page. | ||
|
Replacing -OH by iodine In this case the alcohol is reacted with a mixture of sodium or potassium iodide and concentrated phosphoric(V) acid, H3PO4, and the iodoalkane is distilled off. The mixture of the iodide and phosphoric(V) acid produces hydrogen iodide which reacts with the alcohol.
Phosphoric(V) acid is used instead of concentrated sulphuric acid because sulphuric acid oxidises iodide ions to iodine and produces hardly any hydrogen iodide. A similar thing happens to some extent with bromide ions in the preparation of bromoalkanes, but not enough to get in the way of the main reaction. There is no reason why you couldn't use phosphoric(V) acid in the bromide case instead of sulphuric acid if you wanted to. | ||
|
Note: If you are interested in the reactions between halide ions and concentrated sulphuric acid you could follow this link. In the present context, all you would need to do is read the beginning of that page. If you choose to follow this link, use the BACK button on your browser to return to this page. | ||
|
Reacting alcohols with phosphorus halides Reaction with phosphorus(III) chloride, PCl3 Alcohols react with liquid phosphorus(III) chloride (also called phosphorus trichloride) to make chloroalkanes.
Reaction with phosphorus(V) chloride, PCl5 Solid phosphorus(V) chloride (phosphorus pentachloride) reacts violently with alcohols at room temperature, producing clouds of hydrogen chloride gas. It isn't a good choice as a way of making chloroalkanes, although it is used as a test for -OH groups in organic chemistry. To show that a substance was an alcohol, you would first have to eliminate all the other things which also react with phosphorus(V) chloride. For example, carboxylic acids (containing the -COOH group) react with it (because of the -OH in -COOH), and so does water (H-OH). If you have a neutral liquid not contaminated with water, and get a violent reaction producing clouds of steamy fumes of hydrogen chloride when you add phosphorus(V) chloride, then you have an alcohol group present.
There are also side reactions involving the POCl3 reacting with the alcohol. Other reactions involving phosphorus halides Instead of using phosphorus(III) bromide or iodide, the alcohol is usually heated under reflux with a mixture of red phosphorus and either bromine or iodine. The phosphorus first reacts with the bromine or iodine to give the phosphorus(III) halide.
These then react with the alcohol to give the corresponding halogenoalkane which can be distilled off.
Reacting alcohols with sulphur dichloride oxide (thionyl chloride) The reaction Sulphur dichloride oxide (thionyl chloride) has the formula SOCl2. Traditionally, the formula is written as shown, despite the fact that the modern name writes the chlorine before the oxygen (alphabetical order). The sulphur dichloride oxide reacts with alcohols at room temperature to produce a chloroalkane. Sulphur dioxide and hydrogen chloride are given off. Care would have to be taken because both of these are poisonous.
Why this reaction is useful The big advantage that this reaction has over the use of either of the phosphorus chlorides is that the two other products of the reaction (sulphur dioxide and HCl) are both gases. That means that they separate themselves from the reaction mixture.
© Jim Clark 2003 (modified October 2015) |
||