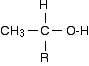|
THE TRIIODOMETHANE (IODOFORM) REACTION WITH ALCOHOLS This page looks at how the triiodomethane (iodoform) reaction can be used to identify the presence of a CH3CH(OH) group in alcohols. | ||
|
Note: This reaction can also be used in testing for the CH3CO group in aldehydes and ketones. You will find a link to this at the bottom of the page. | ||
|
Doing the triiodomethane (iodoform) reaction There are two apparently quite different mixtures of reagents that can be used to do this reaction. They are, in fact, chemically equivalent. | ||
|
Note: It would be silly to learn both of these methods. Use whichever one your examiners want - find out by looking at past papers and mark schemes. If you haven't got these, go to the syllabuses page to find out how to get hold of them. Use the BACK button on your browser to return to this page. | ||
|
Using iodine and sodium hydroxide solution This is chemically the more obvious method. Iodine solution is added to a small amount of an alcohol, followed by just enough sodium hydroxide solution to remove the colour of the iodine. If nothing happens in the cold, it may be necessary to warm the mixture very gently. A positive result is the appearance of a very pale yellow precipitate of triiodomethane (previously known as iodoform) - CHI3. Apart from its colour, this can be recognised by its faintly "medical" smell. It is used as an antiseptic on the sort of sticky plasters you put on minor cuts, for example. Using potassium iodide and sodium chlorate(I) solutions Sodium chlorate(I) is also known as sodium hypochlorite. Potassium iodide solution is added to a small amount of an alcohol, followed by sodium chlorate(I) solution. Again, if no precipitate is formed in the cold, it may be necessary to warm the mixture very gently. The positive result is the same pale yellow precipitate as before. | ||
|
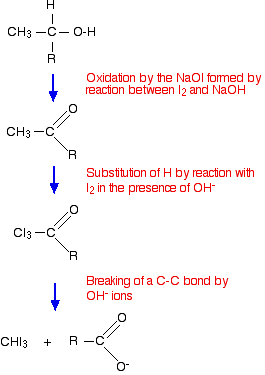
Why the two reactions are equivalent: This reaction happens in three stages. In the first, the alcohol is oxidised to an aldehyde or ketone. In the first mixture, the iodine reacts with the sodium hydroxide solution to produce some sodium iodate(I). This is an oxidising agent. In the second mixture, the sodium chlorate(I) already present is an oxidising agent. After that the reaction happens in two further stages: first the aldehyde or ketone formed reacts with iodine, and the product of that reaction reacts with hydroxide ions. Iodine and sodium hydroxide is exactly what you are adding in the first method above. In the second method, the sodium chlorate(I) solution is an oxidising agent, and oxidises the iodide ions in the potassium iodide to iodine. As well as any possible precipitate, you will also see the typical reddish-brown colour of iodine solution being formed during the reaction. So although you didn't put any iodine into the mixture, it is made in situ. What about the hydroxide ions? Sodium chlorate(I) solution is alkaline and contains enough hydroxide ions to carry out the second half of the reaction. Sodium chlorate(I) is alkaline because it reacts reversibly with water to form the weak acid chloric(I) acid together with hydroxide ions. | ||
|
The chemistry of the triiodomethane (iodoform) reaction What the triiodomethane (iodoform) reaction shows A positive result - the pale yellow precipitate of triiodomethane (iodoform) - is given by an alcohol containing the grouping:
"R" can be a hydrogen atom or a hydrocarbon group (for example, an alkyl group). If "R" is hydrogen, then you have the primary alcohol ethanol, CH3CH2OH.
Summary of the reactions during the triiodomethane (iodoform) reaction We will take the reagents as being iodine and sodium hydroxide solution.
This is being given as a flow scheme rather than full equations. You aren't likely to need the equation for the oxidation stage for UK A level purposes. The equations for the other two steps are given on a page about reactions of aldehydes and ketones. Follow the first link below if you are interested.
© Jim Clark 2004 (modified November 2015) |
||