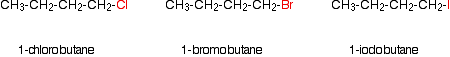|
REACTIONS INVOLVING HALOGENOALKANES AND SILVER NITRATE SOLUTION This page looks at how silver nitrate solution can be used as part of a test for halogenoalkanes (haloalkanes or alkyl halides), and also as a means of measuring their relative reactivities. Testing for halogenoalkanes Silver nitrate solution can be used to find out which halogen is present in a suspected halogenoalkane. The most effective way is to do a substitution reaction which turns the halogen into a halide ion, and then to test for that ion with silver nitrate solution. Doing the reaction The halogenoalkane is warmed with some sodium hydroxide solution in a mixture of ethanol and water. Everything will dissolve in this mixture and so you can get a good reaction. The halogen atom is displaced as a halide ion:
| |||||||||||||||||
|
Note: This reaction is described in more detail on the page about reactions of halogenoalkanes with hydroxide ions. Use the BACK button on your browser to return to this page. | |||||||||||||||||
|
There is no need to make this reaction go to completion. The silver nitrate test is sensitive enough to detect fairly small concentrations of halide ions. The mixture is acidified by adding dilute nitric acid. This prevents unreacted hydroxide ions reacting with the silver ions to give a confusing precipitate. Then silver nitrate solution is added. Various precipitates may be formed from the reaction between the silver and halide ions:
Confirming the precipitates It is actually quite difficult to distinguish between these colours, especially if there isn't much precipitate. You can sort out which precipitate you have by adding ammonia solution.
| |||||||||||||||||
|
Note: You can read more about these tests (including an explanation of this reaction involving ammonia) by following this link to the page about testing for halide ions. Use the BACK button on your browser to return to this page. | |||||||||||||||||
|
Comparing halogenoalkane reactivities Background In this case, various halogenoalkanes are treated with a solution of silver nitrate in a mixture of ethanol and water. Nothing else is added. After varying lengths of time precipitates appear as halide ions (produced from reactions of the halogenoalkanes) react with the silver ions present. As long as you are doing everything under controlled conditions (same amounts of everything, same temperature and so on), the time taken gives a good guide to the reactivity of the halogenoalkanes - the quicker the precipitate appears, the more reactive the halogenoalkane. The halide ion is formed in one of two ways, depending on the type of halogenoalkane you have present - primary, secondary or tertiary. | |||||||||||||||||
|
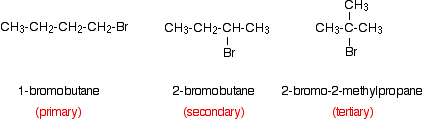
Note: If you aren't sure what primary, secondary and tertiary halogenoalkanes are, you should read the beginning of the introduction to halogenoalkanes by following this link before you go on. Use the BACK button on your browser to return to this page. | |||||||||||||||||
|
For a primary halogenoalkane, the main reaction is one between the halogenoalkane and water in the solvent.
A tertiary halogenoalkane ionises to a very small extent of its own accord.
Secondary halogenoalkanes do a bit of both of these. Comparing the reaction rates as you change the halogen You would have to keep the type of halogenoalkane (primary, secondary or tertiary) constant, but vary the halogen. You might, for example, compare the times taken to produce a precipitate from this series of primary halogenoalkanes:
Obviously, the time taken for a precipitate of silver halide to appear will depend on how much of everything you use and the temperature at which the reaction is carried out. But the pattern of results is always the same. For example:
The order of reactivity reflects the strengths of the carbon-halogen bonds. The carbon-iodine bond is the weakest and the carbon-chlorine the strongest of the three bonds. In order for a halide ion to be produced, the carbon-halogen bond has to be broken. The weaker the bond, the easier that is. If you have looked at the mechanisms for these reactions, you will know that a lone pair on a water molecule attacks the slightly positive carbon atom attached to the halogen. It is slightly positive because most of the halogens are more electronegative than carbon, and so pull electrons away from the carbon. It is tempting to think that the reaction will be faster if the electronegativity difference is greater. The slight positive charge on the carbon will be larger if it is attached to a chlorine atom than to an iodine atom. That means that there will be more attraction between a lone pair on the water and a carbon atom attached to a chlorine atom than if it was attached to an iodine atom. The electronegativity difference between carbon and iodine is negligible. However, the fastest reaction is with an iodoalkane. In these reactions, bond strength is the main factor deciding the relative rates of reaction. Comparing the reaction rates of primary, secondary and tertiary halogenoalkanes You would need to keep the halogen atom constant. It is common to use bromides because they have moderate reaction rates. You could, for example, compare the reactivity of these compounds:
Again, the actual times taken will vary with reaction conditions, but the pattern will always be the same. For example:
It is more difficult to explain the reason for this, because it needs a fairly intimate knowledge of the mechanisms involved in the reactions. It reflects the change in the way that the halide ion is produced as you go from primary to secondary to tertiary halogenoalkanes. | |||||||||||||||||
|
Note: You can read about the different mechanisms for this reaction in the mechanism section of this site if you are interested. Use the BACK button on your browser to return to this page. | |||||||||||||||||
© Jim Clark 2003 (last modified October 2015) |
|||||||||||||||||