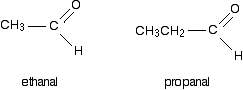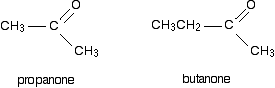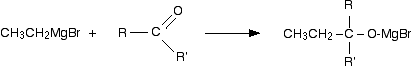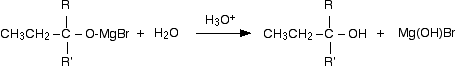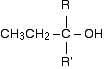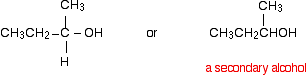|
AN INTRODUCTION TO GRIGNARD REAGENTS This page takes an introductory look at how Grignard reagents are made from halogenoalkanes (haloalkanes or alkyl halides), and introduces some of their reactions. Making Grignard reagents What are Grignard reagents? A Grignard reagent has a formula RMgX where X is a halogen, and R is an alkyl or aryl (based on a benzene ring) group. For the purposes of this page, we shall take R to be an alkyl group. A typical Grignard reagent might be CH3CH2MgBr. The preparation of a Grignard reagent Grignard reagents are made by adding the halogenoalkane to small bits of magnesium in a flask containing ethoxyethane (commonly called diethyl ether or just "ether"). The flask is fitted with a reflux condenser, and the mixture is warmed over a water bath for 20 - 30 minutes.
Everything must be perfectly dry because Grignard reagents react with water (see below). | ||
|
Warning! Ethoxyethane (ether) is very dangerous to work with. It is a liquid with a boiling point of only 34.5°C, and is extremely flammable. It is also an anaesthetic. Under no circumstances should you try to carry out this reaction without properly qualified guidance. (I was challenged by a reader because I had previously used the word "inflammable" rather than "flammable" in this paragraph. In fact, confusingly, the two words mean exactly the same thing. However, by switching to "flammable", I have removed any possible confusion.) | ||
|
Any reactions using the Grignard reagent are carried out with the mixture produced from this reaction. You can't separate it out in any way. Reactions of Grignard reagents Grignard reagents and water Grignard reagents react with water to produce alkanes. This is the reason that everything has to be very dry during the preparation above. For example:
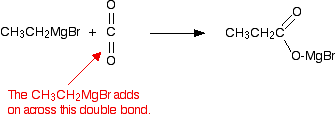
The inorganic product, Mg(OH)Br, is referred to as a "basic bromide". You can think of it as a sort of half-way stage between magnesium bromide and magnesium hydroxide. Grignard reagents and carbon dioxide Grignard reagents react with carbon dioxide in two stages. In the first, you get an addition of the Grignard reagent to the carbon dioxide. Dry carbon dioxide is bubbled through a solution of the Grignard reagent in ethoxyethane, made as described above. For example:
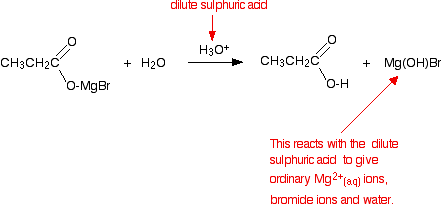
The product is then hydrolysed (reacted with water) in the presence of a dilute acid. Typically, you would add dilute sulphuric acid or dilute hydrochloric acid to the solution formed by the reaction with the CO2. A carboxylic acid is produced with one more carbon than the original Grignard reagent. The usually quoted equation is (without the red bits):
Almost all sources quote the formation of a basic halide such as Mg(OH)Br as the other product of the reaction. That's actually misleading because these compounds react with dilute acids. What you end up with would be a mixture of ordinary hydrated magnesium ions, halide ions and sulphate or chloride ions - depending on which dilute acid you added. | ||
|
Note: What you need to learn about this depends on what your examiners want. The only way to find that out is to look at old exam papers and mark schemes. If you are a UK A level student and haven't got copies of these, find out how to get hold of them by going to the syllabuses page to find your Exam Board's web address. | ||
|
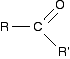
Grignard reagents and carbonyl compounds What are carbonyl compounds? Carbonyl compounds contain the C=O double bond. The simplest ones have the form:
R and R' can be the same or different, and can be an alkyl group or hydrogen. | ||
|
Note: Other carbonyl compounds also react with Grignard reagents, but these are all that are required for UK A level purposes. | ||
|
If one (or both) of the R groups are hydrogens, the compounds are called aldehydes. For example:
If both of the R groups are alkyl groups, the compounds are called ketones. Examples include:
The general reaction between Grignard reagents and carbonyl compounds The reactions between the various sorts of carbonyl compounds and Grignard reagents can look quite complicated, but in fact they all react in the same way - all that changes are the groups attached to the carbon-oxygen double bond. It is much easier to understand what is going on by looking closely at the general case (using "R" groups rather than specific groups) - and then slotting in the various real groups as and when you need to. The reactions are essentially identical to the reaction with carbon dioxide - all that differs is the nature of the organic product. In the first stage, the Grignard reagent adds across the carbon-oxygen double bond:
Dilute acid is then added to this to hydrolyse it. (I am using the normally accepted equation ignoring the fact that the Mg(OH)Br will react further with the acid.)
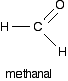
An alcohol is formed. One of the key uses of Grignard reagents is the ability to make complicated alcohols easily. What sort of alcohol you get depends on the carbonyl compound you started with - in other words, what R and R' are. The reaction between Grignard reagents and methanal In methanal, both R groups are hydrogen. Methanal is the simplest possible aldehyde.
Assuming that you are starting with CH3CH2MgBr and using the general equation above, the alcohol you get always has the form:
Since both R groups are hydrogen atoms, the final product will be:
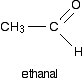
A primary alcohol is formed. A primary alcohol has only one alkyl group attached to the carbon atom with the -OH group on it. You could obviously get a different primary alcohol if you started from a different Grignard reagent. The reaction between Grignard reagents and other aldehydes The next biggest aldehyde is ethanal. One of the R groups is hydrogen and the other CH3.
Again, think about how that relates to the general case. The alcohol formed is:
So this time the final product has one CH3 group and one hydrogen attached:
A secondary alcohol has two alkyl groups (the same or different) attached to the carbon with the -OH group on it. You could change the nature of the final secondary alcohol by either:
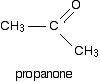
The reaction between Grignard reagents and ketones Ketones have two alkyl groups attached to the carbon-oxygen double bond. The simplest one is propanone.
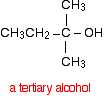
This time when you replace the R groups in the general formula for the alcohol produced you get a tertiary alcohol.
A tertiary alcohol has three alkyl groups attached to the carbon with the -OH attached. The alkyl groups can be any combination of same or different. You could ring the changes on the product by
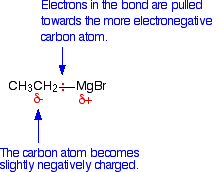
Why do Grignard reagents react with carbonyl compounds? The mechanisms for these reactions aren't required by any UK A level syllabuses, but you might need to know a little about the nature of Grignard reagents. The bond between the carbon atom and the magnesium is polar. Carbon is more electronegative than magnesium, and so the bonding pair of electrons is pulled towards the carbon. That leaves the carbon atom with a slight negative charge.
| ||
|
Note: If you aren't sure about electronegativity, you can read about it in an organic context by following this link. Use the BACK button on your browser to return to this page. | ||
|
The carbon-oxygen double bond is also highly polar with a significant amount of positive charge on the carbon atom. The nature of this bond is described in detail elsewhere on this site. | ||
|
Note: If you are interested, you could follow this link to the bonding in a carbonyl group. Reading the last couple of paragraphs on that page would be enough in the present context. Use the BACK button on your browser to return to this page. | ||
|
The Grignard reagent can therefore serve as a nucleophile because of the attraction between the slight negativeness of the carbon atom in the Grignard reagent and the positiveness of the carbon in the carbonyl compound. A nucleophile is a species that attacks positive (or slightly positive) centres in other molecules or ions. | ||
|
Note: I have included this because one of the UK A level syllabuses says that candidates should "recall that Grignard reagents act as nucleophiles". That is all you need to know! The mechanism is more complex than this suggests at first sight, and isn't required. You won't find these mechanisms anywhere on this site. | ||
© Jim Clark 2003 (modified October 2015) |
||