|
QUESTIONS AND COMMENTS Intermolecular bonding and the physical properties of PTFE | |
|
Important: If you have come straight to this page via a search engine, you should be aware that this is one of a number of pages dealing with e-mail questions that I haven't been able to answer to my satisfaction, although I suspect that this version, written in July 2014, is about as good as I am going to be able to make it. | |
|
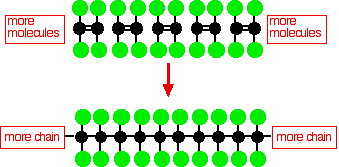
When I was writing about PTFE on the page about polymerisation of alkenes, I spent ages trying to find out why PTFE was non-stick - and failed completely. Part of the information I found on the web I know to be untrue or illogical, but there is a mass of stuff which, to be frank, I simply don't understand. Quite a lot of what is out there is written by physicists or other non-chemists who speak a quite different language from me! It also seems to me that there is a reluctance to start right back at the level of the molecules and explain what is happening in terms of molecular interactions. Following a number of discussions with various knowledgeable people over recent years, what follows is, I hope, logical. Whether it is also the best explanation that can be given is another matter. The structure of PTFE molecules PTFE, poly(tetrafluoroethene), is made by polymerising lots of tetrafluoroethene molecules.
This simple diagram for PTFE doesn't show the 3-dimensional structure of the molecule. In the simpler molecule poly(ethene) the carbon backbone of the molecule just has hydrogen atoms attached to it, and the chain is very flexible - it definitely isn't a straight molecule. However, in PTFE, the fluorine atoms in one CF2 group are big enough to interfere with those on the neighbouring groups. You need to remember that each fluorine atom will have 3 lone pairs sticking out from it. The effect of this is to inhibit rotation about the carbon-carbon single bonds. The fluorine atoms will tend to line up so that they are as far apart as possible from neighbouring fluorines. Rotation will tend to involve a clash of lone pairs between fluorines on adjacent carbon atoms - and this makes rotation energetically unfavourable. The repulsions lock the molecules into a rod-like shape with the fluorines arranged into very gentle spirals - a helical arrangement of the fluorines around the carbon backbone. The rods will then tend to pack together a bit like long thin pencils in a box. This closely touching arrangement has an important effect on the intermolecular forces as you will see. | |
|
Note: Actually, this is a simplification. You will get some kinking in the chains especially as temperature is increased. | |
|
Intermolecular forces and the melting point of PTFE The melting point of PTFE is quoted as 327°C. That's quite high for a polymer of this sort - so there must be sizeable van der Waals forces between the molecules. But . . . several web sites talk about PTFE having very weak van der Waals forces. If it had very weak van der Waals forces, it would be a gas - not a fairly high melting point solid. So we have a problem here! Why do people claim the van der Waals forces in PTFE are weak? van der Waals dispersion forces are caused by temporary fluctuating dipoles set up as electrons in the molecules move around. Since PTFE molecules are large, you would expect the dispersion forces to be large as well, because there are a lot of electrons which can move. It is generally the case that the bigger the molecule, the greater the dispersion forces. However, there is a problem with PTFE. Fluorine is so electronegative that it tends to hold the electrons in the carbon-fluorine bonds closely to itself - so closely that the electrons are prevented from moving as much as you would expect. We describe the carbon-fluorine bonds as not being very polarisable. van der Waals forces also include dipole-dipole interactions. But in PTFE each molecule is sheathed in a layer of slightly negative fluorine atoms. The only interactions possible between molecules in this case are repulsions! So the dispersion forces are weaker than you might expect, and dipole-dipole interactions are going to tend to cause repulsion. It is no wonder that people claim that van der Waals forces are weak in PTFE. You don't actually get repulsion because the effect of the dispersion forces outweighs that of the dipole-dipole interactions, but the net effect is that the van der Waals forces will tend to be weak. And yet PTFE has a high melting point, and so the forces holding the molecules together must be strong. How can PTFE have a high melting point? PTFE is very crystalline in the sense that there are large areas where the molecules are lying in a very regular arrangement. Remember that PTFE molecules can be thought of as long thin rods. These rods will pack very closely together. That means that although PTFE molecules can't generate really big temporary dipoles, the dipoles that are produced can be used extremely effectively. So are the van der Waals forces in PTFE weak or strong? I think you could argue it both ways! If you had PTFE chains arranged in such a way that the chains didn't have much close contact, then the forces between them would be weak, and the melting point would be low. But in the real world, the molecules are closely touching. The van der Waals forces may not be as strong as they could be, but the structure of the PTFE means that they are felt to the maximum effect, producing overall strong intermolecular bonding and a high melting point. Update May 2020 It was pointed out to me that dispersion forces fall off very rapidly with distance - much more rapidly than other intermolecular forces. If you double the distance between molecules, dispersion forces fall by a factor of 26, or 64 times. That contrasts with other forces like dipole-dipole interactions which fall off only by a factor of 23, or 8 times for a doubling of distance. So the close packing of the rod-like molecules in PTFE maximises the effectiveness of the dispersion forces. My thanks to Christopher Grayce for this insight. Non-stick properties and friction Virtually every site that I have looked at treats the relative lack of friction of PTFE and its non-stick properties as if they were the same effect. I don't think that's true. The non-stick properties This is about why things like water and oil don't stick to the surface of PTFE, and why you can fry an egg in a PTFE-coated pan without lots of it ending up stuck to the pan. You need to consider what forces might hold other molecules to the PTFE surface. Possibilities might include some sort of chemical bonding, van der Waals forces or hydrogen bonds. Chemical bonding Carbon-fluorine bonds are very strong, and there is no way that any other molecule could get at the carbon chain to enable any sort of substitution reaction to take place. No sort of chemical bonding could take place. van der Waals forces We've seen that the van der Waals forces in PTFE aren't very strong, and only work to give PTFE a high melting point because the molecules lie so close together and there is very effective contact between them. But it is different for other molecules approaching the surface of the PTFE. A relatively small molecule (like a water or an oil molecule) will only have a small amount of contact with the surface, and will only produce a small amount of van der Waals attraction. A large molecule (like a protein, for example) isn't going to be rod-like and so, again, there isn't going to be enough effective contact between it and the surface to overcome the low tendency of the PTFE to polarise. Either way, van der Waals forces between the PTFE surface and whatever is around it are going to be small and ineffective. | |
|
Note: As a similar example, it has been pointed out to me (February 2015) that long-chain perfluoroalkanes (big alkanes in which all the hydrogens have been replaced by fluorine atoms) can form a third phase if they are mixed with water and a hydrocarbon solvent. Because they are so weakly attracted by both, they form a third layer instead of dissolving in one or the other. | |
|
Hydrogen bonds The PTFE molecules on its surface are completely encased in fluorine atoms. Those fluorine atoms are very electronegative and so will all carry some degree of negative charge. Each fluorine also has three lone pairs of electrons sticking out. Those are exactly the conditions needed for hydrogen bonding to be possible between lone pairs on the fluorines and hydrogen atoms in water for example. But it clearly doesn't happen - otherwise there would be strong attractions between PTFE molecules and water molecules and water would stick to the PTFE. In November 2013, an Iranian PhD student pointed out to me a 1997 paper by Dunitz and Taylor with a title "Organic Fluorine Hardly Ever Accepts Hydrogen Bonds". If you are interested, you can find it from this site if you have the right access. They found that only a tiny number of compounds containing C-F bonds would form hydrogen bonds, whereas compounds like HF or the F- ion formed strong hydrogen bonds. What they didn't come up with, however, was any definite explanation for this, although they suggested that a possible explanation could lie in the fact that the fluorine atom holds its electrons very tightly in towards the nucleus, and as a result the C-F bond isn't very polarisable. The electrons won't move sufficiently towards a hydrogen from water (or anything similar) in order for a hydrogen bond to form. Personally, I have a problem seeing why that is different from the situation in H-F or a fluoride ion, both of which can form hydrogen bonds with water. And their final sentence said:
Summary There are no available methods for other molecules to attach themselves successfully to the surface of the PTFE, and so it is has a non-stick surface. The low friction PTFE has a very low coefficient of friction. What this means is that if you have a surface coated with PTFE, other things will slide on it very easily. What follows is just a quick summary of what is happening. This comes from a 1992 paper called Friction and wear of PTFE - a review which is available free from this link.
What holds the PTFE layer onto the substance it is sliding against is quite complicated, and thoughts on this may have changed since the paper was written. If you are interested, you will find it discussed on the page numbered as 203 of the paper (page 11 of 19 on my pdf reader). Acknowledgements I would like to thank Timothy Duncan for helping to sort out my thoughts about why PTFE has a high melting point, and Masoud Sadeghi for the useful lead about the lack of hydrogen bonding in fluorines joined to carbon. Return to questions list . . . © Jim Clark 2007 (major re-write July 2014. Last modified May 2020) |
|