|
Chemguide: Core Chemistry 14 - 16 An introduction to electrolysis This page introduces the key words and ideas in electrolysis. You will find detailed examples of electrolysis in other pages in this section. What is electrolysis? Electrolysis is defined as
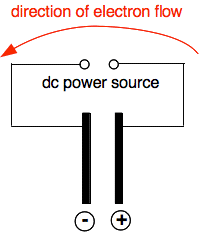
Essential ideas about electricity Electric currents A flow of electricity through a metal or carbon is simply a flow of electrons. In a metal, the electrons flowing are the delocalised electrons (also known as a "sea of electrons") which hold the metal together. If you aren't confident about metallic bonding then have a look at the metallic bonding page before you go on. In a carbon (graphite) electrode, there are also delocalised electrons which can move through the structure. If you want a description of this structure you could look at this page. Batteries and other power sources The simplest way of thinking about a battery or other similar power source is as an electron pump. Suppose you connected two bits of metal or carbon to a direct current (dc) power source of some kind. The power source will pump electrons from one to piece to the other.
How many electrons get pumped depends on the strength of the power source. As you pump more electrons on to the left-hand piece of metal in the diagram, it becomes negatively charged. Eventually, the incoming electrons just get repelled by the ones already there and there is no further increase in negativity. Electrons are beng pumped away from the right-hand side, and that shortage of electrons leaves it positively charged. If you put a battery or ammeter into the circuit, nothing would happen to either. After the small virtually instantaneous movement of electrons at the beginning, there is no further current flow. Using this simple circuit for electrolysis Key words The electrodes are the pieces of metal or carbon I have shown as black rods in the diagram above. In most cases you will come across, these aren't changed by the electrolysis, and we describe them as "inert". Most of the time in the lab, you use carbon (graphite) electrodes, but occasionally you might come across platinum being used. The positive electrode is called the anode. The negative electrode is called the cathode. It is really important that you get these the right way round! There is a simple way of remembering it. If you take the initial letters of Positive, Anode, Negative and Cathode, there are only a few ways of arranging these that make a sayable word. The simplest of these is PANC - positive anode, negative cathode. Learn that! PANC - positive anode, negative cathode. | |
|
Note: CANP is also a sayable word and gives the same information - cathode, anode in the order negative, positive. But that's more of an effort to work out. You can sort of say CNAP as well - that's just PANC written backwards and gives the same information. PANC is easiest - learn it. | |
|
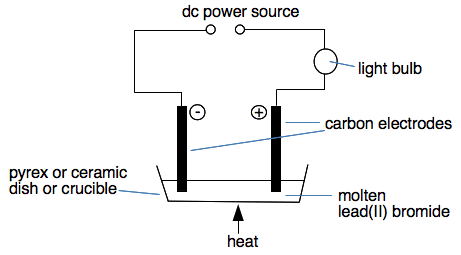
The electrolyte is the substance undergoing electrolysis. This will be an ionic compound either molten or in solution. As you will see later, electrolysis involves the movement of ions towards the electrodes. Ions can't move in a solid. Positive ions move towards the negative cathode during electrolysis. Positive ions are known as cations. Negative ions move towards the positive anode during electrolysis. Negative ions are known as anions. In the early stages, it is better to work out what an anion and a cation are than to remember it. For example, it is obvious that a positive ion will move towards the negative cathode - so it is called a cation. It is named after where it ends up. What happens during electrolysis? Most teachers and books will probably introduce electrolysis by electrolysing molten lead(II) bromide because it is easy to do, and visually obvious what is going on. Overall, electrolysis splits lead(II) bromide into lead and bromine.
Note the state symbols. This is done very hot so that the lead(II) bromide is molten as is the lead which is formed. The bromine comes off as a gas. The apparatus would look like this:
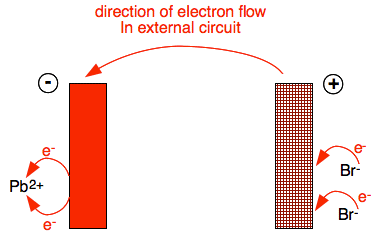
If we look at what is happening at the two electrodes in detail:
The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. Positive lead(II) ions are attracted to it. When they get there, 2 electrons jump off the electrode and cancel the charge on the lead(II) ion. A lead atom is formed.
Molten lead will collect under the electrode. The anode (the positive electrode) has electrons being pumped away from it, making it positive. Bromide ions will be attracted to it. When they get there, an electron jumps off each bromide ion to leave a bromine atom. But bromine atoms form molecules, Br2. So the two atoms of bromine shown being formed in the diagram join up to form Br2, which comes off as a brown gas.
What is happening in the circuit? Every unit of PbBr2 which is discharged in this way, puts two new electrons on to the anode, and takes two electrons off the cathode. The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. A movement of electrons is an electric current - so electricity is now flowing in the external circuit.
If you think about the system as a whole, electricity is being carried by electrons in the external circuit, but by the movement and discharge of ions in the electrolyte. This can only work if you have ions which are free to move. That is why an electrolyte has to be an ionic compound, either molten or in solution. This is a fairly uncomplicated electrolysis to explain, but all electrolysis reactions work on the same principles. We will look at other examples on other pages. Oxidation and reduction in electrolysis This next bit assumes that you understand oxidation and reduction in terms of electron transfer. If you aren't happy about this, follow this link before you go on. Look again at the electrode reactions we have just written above. At the cathode
The lead(II) ions are gaining electrons. Gain of electrons is reduction (OIL RIG). The reaction happening at the cathode is reduction. It doesn't matter what you are electrolysing - that will always be true. Every cathode reaction involves something gaining electrons. At the anode
Bromide ions are losing electrons at the anode. Loss of electrons is oxidation (OIL RIG again). Again this is true for every electrolysis. The reaction happening at the anode is always oxidation. Every anode reaction involves something losing electrons.
© Jim Clark 2020 |
|