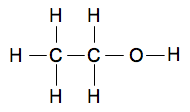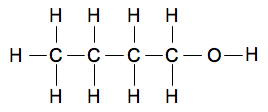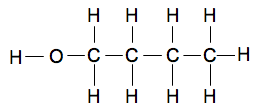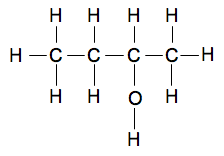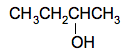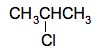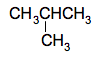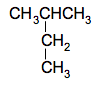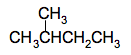|
Chemguide: Core Chemistry 14 - 16 Understanding how organic compounds are named The names of organic compounds can look quite scary when you start, but there is a simple logic behind them. You have to do a tiny bit of learning and then everything will fall into place. I am assuming that you have already read the page about organic formulae. Why is ethanol called ethanol? Modern organic names are simply codes for the structures. Here is the fully displayed formula for ethanol:
You can split the name into three bits: "eth", "an" and "ol".
So if you knew that, you could construct the structural formula. You would draw the carbon chain, leaving the bonds with nothing attached to them for the moment.
Now you can attach an OH group to one of the bonds.
Finally you can fill any unused bonds with hydrogen atoms.
| |||||||||||||||||||||||||
|
Note: Does it matter which of the bonds in the first diagram you attach the OH group to? No! All the hydrogen positions are equivalent. If you aren't sure about that, look at the model for ethane, C2H6, on the page about how organic formulae. If you are drawing a structure with an interesting group on it, you normally write it on the right-hand end unless the name tells you differently. We will look at that in a moment. | |||||||||||||||||||||||||
|
Codes for chain length These are based on the names for a group of hydrocarbons called alkanes. The first 6 alkanes are:
So the codes for chains containing up to 6 carbon atoms are:
You have to learn these. The first four don't have any obvious logic; the last two do. You are probably familiar with a pentagon which has 5 sides, and hexagon which has 6. | |||||||||||||||||||||||||
|
Note: At this point you need to check your syllabus. Some syllabuses only expect you to name chains up to 4 carbons; others expect you to learn the first 6. It obviously pays you to know what you have got to do! | |||||||||||||||||||||||||
|
Butan-1-ol and butan-2-ol Butan-1-ol On the page about drawing various types of structures, I mentioned in passing butan-1-ol. This introduces a new factor - names which have numbers in. The number refers to the number of the carbon atom the OH group is attached to. You always number the chain to produce the smallest possible number(s) in the name. So going through the same process again:
So, putting that together, you get:
We normally write the OH group at the right-hand end of the molecule, but if for some reason you chose to write it at the left-hand end, you have to be careful. It is the oxygen that is bonded to the carbon and not the hydrogen. You have to remember to flip the OH group around, otherwise it is wrong.
Butan-2-ol If you missed the 1 out of butan-1-ol, that would be confusing, because the OH group could just as well be attached to one of the carbon atoms in the middle of the chain. That's butan-2-ol.
If you attached the OH group to the other central carbon atom, that is exactly the same compound - you have just flipped it over end-to-end. Remember that you always number the chain from the end which produces the smallest numbers in the name. If you were writing the normal simplified structural formula for this, it would be CH3CH2CH(OH)CH3 If you wanted to make it clearer, you could just write the chain as CH3CH2CHCH3 and draw a vertical line up or down from the CH group to an OH group.
Attaching chlorine or bromine atoms to a chain You show these by writing "chloro" or "bromo" at the beginning if the name. For example, chloromethane is CH3Cl - chlorine atom attached to a 1 carbon "chain". Bromoethane is CH3CH2Br - a bromine attached to a two carbon chain with a single carbon-carbon bond. If you have 3 or more carbon atoms in the chain, you have to use numbers to tell you which carbon atom the chlorine or bromine is attached to. For example, 1-chloropropane is CH3CH2CH2Cl. 2-chloropropane is CH3CHClCH3, or more clearly:
You will come across a case later where there are 2 bromine atoms attached to a short chain of 2 carbon atoms. In that case the name will tell you both that there are 2 bromine atoms and where they are. The compound in question is 1,2-dibromoethane, CH2BrCH2Br. The name tells you that there are two bromine atoms, one on the number 1 carbon and one on the number 2 carbon. Branched carbon chains Let's look at some of the alkanes that I listed above. Those with 4 or more carbons can take more than one structural form. For example, the molecular formula C4H10 could be either CH3CH2CH2CH3 or
The CH3 group can be thought of as a methane molecule, CH4, which has lost a hydrogen atom. It is called a methyl group. The name of this compound is 2-methylpropane. The longest chain has 3 carbon atoms and so the name is based on "prop", and the methyl group is attached to the number 2 carbon atom. Other similar groups you might come across are ethyl, CH3CH2, propyl, CH3CH2CH2, butyl, CH3CH2CH2CH2, and so on. These are known collectively as alkyl groups But you have to be careful if you have longer side chains like this. For example, consider this compound:
You might think this would be called 2-ethylpropane - there is an ethyl group attached to the number 2 carbon in a 3-carbon chain. But it's wrong. It is wrong because the longest chain you can find in the molecule is a 4-carbon chain. The rule is that you always draw the longest chain you can find horizontally when you name something. In this case, as
This is a 4-carbon chain with a methyl group attached to the number 2 carbon. So the proper name is 2-methylbutane. These last two structures are exactly the same molecule. You have 4 carbon atoms joined up in a continuous chain with a methyl group attached to a number 2 carbon in the chain. If you made models of them, you could turn one into the other just by twisting various carbon-carbon bonds. | |||||||||||||||||||||||||
|
Note: Don't get too worried about this! I am pointing it out because it might matter when we are talking about isomerism on the next page if your syllabus wants you to work with bigger molecules. Just remember always to draw these structures with the longest chain you can find horizontally. | |||||||||||||||||||||||||
|
Molecules with carbon-carbon double bonds Ethene The simplest of these is ethene, C2H4, which has a double carbon-carbon bond between two CH2 groups: CH2=CH2 If you look at the name, you will see that it no longer contains the "an" code for the compound to have only carbon-carbon single bonds. If a chain contains a carbon-carbon double bond, the code changes to "en" rather than "an". So the name tells you that the longest chain contains two carbon atoms ("eth") and a double bond - which exactly describes the structure above. | |||||||||||||||||||||||||
|
Warning: You have to look at organic names very carefully. A difference of one letter can describe a completely different structure. | |||||||||||||||||||||||||
|
Propene This is a chain of 3 carbons containing a carbon-carbon double bond. CH3CH=CH2 | |||||||||||||||||||||||||
|
Warning: Don't make the mistake of writing the middle carbon as CH2. If you do that, it is apparently forming 5 bonds - 2 to the other double-bonded carbon, 1 to the left-hand carbon, and 2 to the hydrogens. Carbon can't form 5 bonds. It is easy to get this wrong, and you will lose the mark if you do this in an exam. | |||||||||||||||||||||||||
|
Butene In fact there are two different ways of placing a double bond in a 4-carbon chain: CH3CH2CH=CH2 CH3CH=CHCH3 These are called but-1-ene and but-2-ene respectively. The number represents the number of the carbon atom the double bond starts from, remembering that you always choose to number from the end which produces the smallest number(s) in the name. So in the first structure, the double bond starts on the number 1 carbon, and in the second, on the number 2 carbon. Double-bonded compounds containing other things This works in exactly the same way as in the single-bonded cases we looked at before. The only ones you are likely to come across are chloroethene, CH2=CHCl and tetrafluoroethene, CF2=CF2. "Tetra" in a name means four - so 4 fluorines in a 2-carbon chain containing a double bond. If you have 4 fluorines, there isn't any room for any hydrogens. You will meet these when we talk about polymerisation. Chloroethene used to be called vinyl chloride, and is the basis for PVC - polyvinylchloride, now properly called poly(chloroethene). Tetrafluoroethene is the basis for poly(tetrafluoroethene) - usually called PTFE or Teflon, and used in non-stick coatings. Other names There are a couple of other sorts of name you will come across later, but I am going to leave discussing them until you need to know about them. There is already enough on this page for you to deal with in one go. As long as you recognise the fact that the names are a code, and know the basis for the code, it isn't that difficult. The most important skill is being able to turn a simple name into a formula. If an exam question mentions, say, propene or ethanol, and you can't write a formula for it, you are completely stuck if the rest of the question depends on it.
© Jim Clark 2021 |
|||||||||||||||||||||||||