|
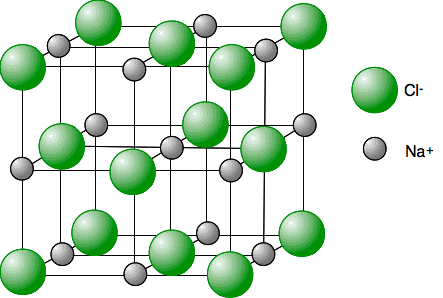
Chemguide: Core Chemistry 14 - 16 How to draw a simple sodium chloride lattice This page is an add-on to a page about giant ionic structures. It simply describes how to draw the structure of a sodium chloride lattice This is the diagram we are aiming at:
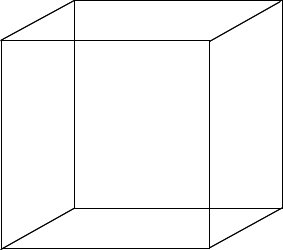
First draw a perfect cube Draw a square:
Then draw an identical square, offset from the first one.
Getting the offset right is important. If you get it wrong, when you draw the ions, they will all end up in a muddle! The only way you are going to find this out is by trial and error. If you take the diagram above as a guide, you won't go far wrong. Now join the two squares together to make a cube.
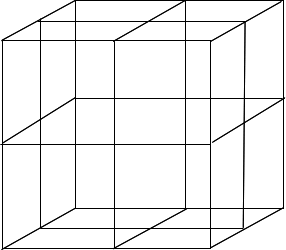
Divide the cube into 8 smaller cubes Start by drawing lines from the mid-points of each edge to the edge opposite on the same face of the cube. When you have done each face, it will look like this:
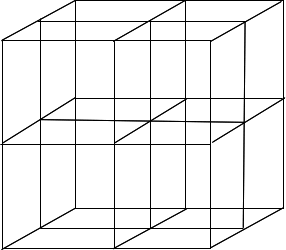
Finally, you have to draw three lines through the centre of the cube from the centre of each face to the centre of the opposite face.
Don't worry if, when you have finished, the lines don't meet exactly, or it looks a bit tatty. The sketches above are definitely flawed! We are doing chemistry, not technical drawing. All the flaws will get covered up when you add the ions. Put the ions on
Don't forget to add a key showing which ion is which. Chloride ions have a diameter which is roughly twice the size of the sodium ions. The exact numbers don't matter - just show the chloride ions as bigger. In the top layer of ions, it doesn't matter whether you start with sodium ions or chloride ions in the corners. All that matters is that you alternate the ions so that you never get two ions the same joined directly by one of the lattice lines. To be sure you know exactly what you are doing, it would probably be a good idea to draw this twice - once with the chloride ions on the corners of the big cube, and once with sodium ions on those corners. That will force you to think rather than just copy. When you have finished, always check that every lattice line has a sodium ion at one end and a chloride ion at the other. If you find two chloride or two sodium ions with a direct line joining them, you have done it wrong! © Jim Clark 2019 |