|
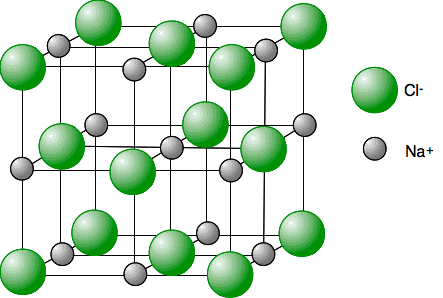
Chemguide: Core Chemistry 14 - 16 Giant ionic structures This page looks at the way ions arrange themselves into giant ionic structures, and the effect this has on their simple properties like melting and boiling points and solubility. I am assuming that you already know about ionic bonding. What is a giant ionic structure? Sodium chloride is a good example of a giant ionic structure made up of a regular lattice of alternating sodium ions, Na+, and chloride ions, Cl-, in three dimensions, extending over huge numbers of ions. If you have read the page on ionic bonding already, you will have come across this model of a tiny part of a sodium chloride lattice.
This shows a typical part of the whole lattice. The sodium ions are the silver balls; the chloride ions the green ones. This is usually drawn in an "exploded" view to show more clearly how the whole thing is arranged.
The lattice goes on and on in all three dimensions over potentially huge numbers of ions. Notice the alternating positive and negative ions in all three dimensions. When we draw these lattices, the lines just pick out the grid pattern of the lattice. They are not covalent bonds. They are not shared pairs of electrons. What holds the lattice together are the attractions between the touching positive and negative ions. If there are lines between two nearby ions, they are touching; if there are no lines between two ions, they aren't. So you will never find direct lines between two ions of the same type. They would repel if they touched. | |
|
Note: You will find instructions on how to draw this structure by following this link.
And you will also find a very short bit of YouTube video showing the relationship between a bigger crystal of sodium chloride and this basic diagram by following this link. | |
|
Simple physical properties of giant ionic compounds Melting and boiling points The forces between positive and negative ions in the lattice are strong and this leads to ionic compounds having high melting and boiling points. For example, sodium chloride and magnesium oxide have identical structures and both have high melting and boiling points.
| |
|
Note: Different sources give different values for these, but they are always about the same - which is all we are really interested in. | |
|
The higher melting and boiling points of magnesium oxide compared with sodium chloride are because in magnesium oxide you have 2+ ions attracting 2- ions - a much stronger attraction than 1+ to 1- in sodium chloride. A final caution on melting and boiling points The choice of sodium chloride and magnesium oxide in this comparison isn't random! They both have exactly the same arrangement of ions in the giant lattice, and neither decomposes (breaks into chemically simpler substances) when they are heated. As you will see when you do some more chemistry, an awful lot of ionic substances do split up when you heat them, and so may never reach their melting or boiling points. Brittleness Giant ionic structures can be quite hard solids, but are very brittle. They are easily broken into pieces. Sodium chloride occurs naturally as rock salt. This was formed as ancient seas dried up and the sodium chloride crystallised out. The photo shows a piece of rock salt:
The appearance of rock salt varies depending on where it came from. The orange-brown colour of this sample comes from silt or other bits of rocky material which settled out from the ancient sea at the same time as the sodium chloride. The rock salt in the next bit of video looks different because the source is different, but it is still mainly sodium chloride. The YouTube video shows how little force is needed to split the original lump into two, and then to crush it further. To explain the brittleness, we need to go back to the salt lattice.
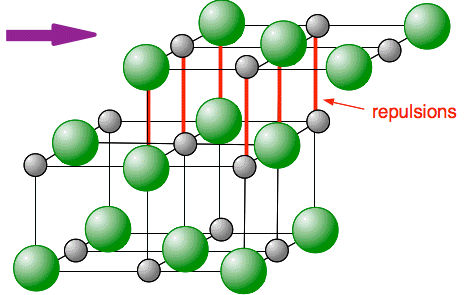
Now suppose you apply a force (a tap from a hammer, for example) so that you shift a layer of the lattice just a small amount.
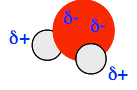
You can see that a small movement of any layer brings ions of the same charge next to each other. You get massive repulsion, and the crystal would fall to pieces where that shift has happened. Solubility in water Soluble ionic compounds such as sodium chloride To break a sodium chloride lattice into moving sodium and chloride ions, you have to heat it to at least 801°C to melt it. On the other hand, if you just stir some salt in water at room temperature, it dissolves, splitting up into separate sodium ions and chloride ions in solution. How can that happen? To break bonds needs energy, and that must be being supplied from somewhere. What is happening is that new attractions are being set up between the ions and water molecules - and setting up attractions releases energy. If you have read the page about bond polarity, you will have seen this diagram showing the polarity of a water molecule. (If you haven't read that page, it would pay you to do so before you go on.)
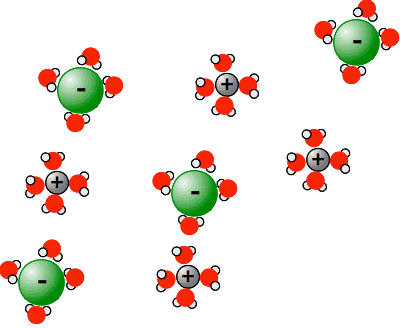
The oxygen is much more electronegative than the hydrogens, and so pulls bonding electrons towards itself. That leaves the oxygen fairly negative, and the hydrogens fairly positive. So water molecules cluster around the ions on the outside of the sodium chloride crystal. The negative chloride ions are attracted to the δ+ hydrogens, and the positive sodium ions are attracted to the δ- oxygens. Formation of these new attractions provides the energy to break the old ones.
Some comments:
There is a simple YouTube animation showing this. It uses three words which might be unfamiliar to you:
Ionic compounds insoluble in water such as magnesium oxide Whether something dissolves in water or not depends on the balance between the strength of the ionic bonds being broken as compared with the strength of the new attractions with the water molecules. In the case of magnesium oxide, the powerful attractions between the 2+ magnesium and 2- oxide ions are very strong. The attractions between the water molecules and the ions aren't strong enough to overcome the forces holding the lattice together - and so the magnesium oxide doesn't dissolve in water. | |
|
Note: Predicting whether a particular ionic substance is going to be soluble in water or not is seriously complicated! I'm not trying to make any generalisations here. There are plenty of ionic substances involving 2+ and 2- ions which do dissolve in water. | |
|
Solubility in organic solvents Organic solvents are ones based on carbon compounds. Common examples include propanone (acetone), hexane (one of the many components of petrol (gasoline)) and tetrachloromethane (carbon tetrachloride). With very few exceptions, ionic compounds won't dissolve in these. Their molecules are either non-polar or not strongly enough polar for them to be able to form any sort of significant attractions to ions. Conduction of electricity When a piece of copper wire conducts electricity, what is happening is that electrons are flowing along it. At no point is the copper changed chemically by this - it is purely a physical property. Solid ionic compounds such as sodium chloride don't conduct electricity - there are no free electrons. But molten sodium chloride, and other meltable ionic compounds, do conduct electricity. However, there is a big difference here from metals. When molten sodium chloride conducts electricity, it splits up into sodium and chlorine - a chemical change takes place. The process is described as electrolysis, and involves the movement of ions in the molten compound - not electrons. All I am doing at the moment is mentioning this. There is a section covering electrolysis which you could take a look at, but it would be better left until later.
© Jim Clark 2019 (up-dated May 2021) |
|