|
Chemguide: Core Chemistry 14 - 16 The electrolysis of aqueous solutions of ionic compounds using inert electrodes This page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. If you haven't recently done so you should first read the pages introducing electrolysis and the electrolysis of molten ionic compounds. Those are an essential introduction before you go on to the more complicated cases of electrolysis in solution. Doing the experiments You can, of course, just dip a couple of carbon electrodes connected to a battery or other power source into a beaker containing the solution. The problem with that is that you can't easily collect and identify any gases given off. There are two methods of getting around this. If you want to collect a reasonable amount of any gases given off, you can do the reaction in a side-arm U-tube.
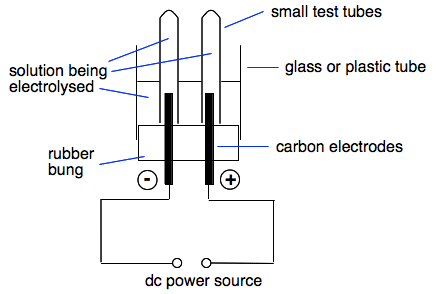
You can attach delivery tubes to the side-arms and collect any gas over water. But if you only want to collect a small amount of gas, there is a neat piece of apparatus designed specifically for the job.
The small tubes are originally filled with the solution you are electrolysing and then inverted over the electrodes. | |||||||||||||||||||||||||||
|
Note: Technically, the tubes shouldn't sit firmly on the rubber bung - you need a free flow of ions between the two electrodes. In practice, it works well enough if the tubes are just resting on the bung. If electrolysis is slow, you can always just lift the tubes a bit. In my experience it isn't necessary to clamp the tubes. | |||||||||||||||||||||||||||
|
Looking at some results - the water problem If you electrolyse dilute sulfuric acid, H2SO4 and, separately, sodium hydroxide solution, NaOH, you get exactly the same result although these are two completely different compounds.
It isn't at all obvious from the formulae that this is what you would get if you electrolysed them. The problem with electrolysing solutions is that water can get involved as well as the ions from the electrolyte. Water molecules ionise slightly to give hydrogen ions and hydroxide ions in solution. The reaction is reversible, and the two-way arrows show that.
I am going to talk about the "forward reaction" and the "back reaction". The forward reaction is the left-to-right reaction, and the back reaction is the one between hydrogen ions and hydroxide ions going from right-to-left. The reactions are happening in both directions all the time. At any one time about 1 in 10 million molecules have ionised. Producing hydrogen from the water There are two different ways of looking at this and which actually happens will vary from case to case. I suggest you learn whichever way your teacher or your examiners prefer and ignore the other one. Getting hydrogen directly from the water
Getting hydrogen from hydrogen ions Suppose two hydrogen ions in the solution picked up an electron each from the negative cathode.
The two hydrogen atoms produced join up to make a hydrogen molecule. That means that the back reaction hasn't happened for those hydrogen ions, but the forward reaction is still happening. The net effect is that for every hydrogen ion discharged during electrolysis, another one is instantly produced from the water ionising. So the fact that there aren't many hydrogen ions in solution at any one time doesn't matter. As fast as they are discharged they are replaced by more water ionising. Producing oxygen from the water There are two different ways of looking at this as well and which actually happens will vary from case to case. Again, learn whichever way your teacher or your examiners want and ignore the other one. Getting oxygen directly from the water
Getting oxygen from hydroxide ions Hydroxide ions from the water can also be discharged during electrolysis. The equation is more complicated here.
Again, for every hydroxide ion that gets discharged another one is produced by the water ionising. | |||||||||||||||||||||||||||
|
Note: For both hydrogen and oxygen, I prefer to use the versions starting from hydrogen ions or hydroxide ions, and that is what I shall do for the rest of this page because it gets too confusing to use both versions side-by-side. | |||||||||||||||||||||||||||
|
How do you decide what is going to be discharged? Positive ions Consider the case of electrolysing sodium hydroxide solution we started from. Hydrogen is produced at the cathode. But both sodium ions and hydrogen ions (from the water) will have been attracted to the cathode. Hydrogen ions are discharged rather than sodium ions because it is easier. It is actually very difficult to persuade a sodium ion to take up an electron to make a sodium atom. Remember the reactivity series:
The higher up the reactivity series you go, the easier it is for the elements to form ions, and the more difficult it is to persuade the ion to pick up an electron again to go back to the atom. So, where there is a choice between discharging a metal ion or discharging a hydrogen ion
| |||||||||||||||||||||||||||
|
Note: It isn't actually quite as simple as that. It is tue for metals below hydrogen, and it is true for the very reactive metals, but for those in between like, say, zinc and lead concentration also matters. If you electrolyse zinc sulfate solution of a reasonable concentration, you do actually get zinc metal formed. At this level, exam questions will normally just ask about clear-cut cases. | |||||||||||||||||||||||||||
|
Negative ions There is a simple rule here as well.
| |||||||||||||||||||||||||||
|
Note: There is also an exception here which we will explore further down the page. If you have a very dilute solution of sodium chloride, for example, you will get oxygen at the anode. A concentrated solution produces chlorine. Some examiners might ask you about this, so we will look at the problem in detail. | |||||||||||||||||||||||||||
|
There is a quick summary of some of this in this video from the BBC: The electrolysis of dilute hydrochloric acid using inert electrodes This video is a bit long, but is a good illustration of how to do a simple electrolysis. At the cathode Only hydrogen ions arrive and these are discharged to form hydrogen gas.
At the anode Chloride ions and hydroxide ions (from the water) arrive. The chloride ions are discharged to form chlorine gas. It so happens that this video uses red litmus to test for the chlorine whereas the previous one used blue litmus. It really doesn't matter - the colour change you want is from coloured to bleached.
We will talk about this in a bit more detail when we look at the electrolysis of sodium chloride solution at the bottom of this page. The electrolysis of dilute sulfuric acid using inert electrodes You can do this using exactly the same apparatus as you have just seen, replacing the hydrochloric acid by dilute sulfuric acid. All the other examples on this page can also be carried out using this same apparatus. At the cathode Only hydrogen ions arrive and these are discharged to form hydrogen gas.
At the anode Sulfate ions and hydroxide ions (from the water) arrive. The sulfate ions are difficult to discharge, and so hydroxide ions are discharged to form oxygen gas.
If you imagine that 4 electrons flow around the circuit, 2 hydrogen molecules would be formed, but only 1 oxygen molecule. So you get twice as much hydrogen as oxygen. The electrolysis of copper(II) sulfate solution using inert electrodes At the cathode Copper(II) ions and hydrogen ions arrive. Copper is below hydrogen in the reactivity series and so is discharged in preference to the hydrogen. A layer of brown copper builds up on the cathode.
At the anode Sulfate ions and hydroxide ions (from the water) arrive. The sulfate ions are difficult to discharge, and so hydroxide ions are discharged to form oxygen gas.
If you let this electrolysis run for a long time, the colour of the copper(II) sulfate fades, and eventually bubbles of hydrogen are formed at the cathode. What has happened is that the copper(II) ions in the solution are being removed and so are hydroxide ions from the water. That leaves hydrogen ions (from the water) and sulfate ions in the solution - sulfuric acid. The electrolysis of silver nitrate solution using inert electrodes At the cathode Silver ions and hydrogen ions arrive. Silver is below hydrogen in the reactivity series and so is discharged in preference to the hydrogen. Silver builds up on the cathode.
At the anode Nitrate ions and hydroxide ions (from the water) arrive. The nitrate ions are difficult to discharge, and so hydroxide ions are discharged to form oxygen gas.
The electrolysis of sodium chloride solution using inert electrodes At the cathode Sodium ions and hydrogen ions arrive. Sodium is above hydrogen in the reactivity series and so hydrogen ions are discharged instead to form hydrogen gas.
At the anode Chloride ions and hydroxide ions (from the water) arrive. The chloride ions are discharged to form chlorine gas.
Warning! This is a bit of a simplification. What you get depends on the concentration of the solution. If you have reasonably concentrated sodium chloride solution, you do in fact get chlorine produced. But if the solution is very dilute, you get oxygen formed from the discharge of the hydroxide ions.
In fact, hydroxide ions are rather easier to discharge than chloride ions. However this is offset by the fact that in any reasonably concentrated solution of sodium chloride there are far, far, more chloride ions than hydroxide ions. So . . .
This also applies to the hydrochloric acid we have looked at before. However, hydrochloric acid as used in the lab is concentrated enough that you will get mainly chlorine. | |||||||||||||||||||||||||||
|
Note: If you are preparing for an exam, you need to find out whether your examiners might ask you about this. Looking at the current UK GCSE syllabuses, it isn't always clear what they want. Some syllabuses specify "concentrated"; some are more vague. If you are doing a UK-based syllabus, you can find links to the Exam Boards' websites where you can download a copy of your syllabus and other useful stuff on the about this part of Chemguide page. If you are using a textbook endorsed by the examiners whose papers you are taking, see what they say. Otherwise take the advice of your teacher. | |||||||||||||||||||||||||||
© Jim Clark 2020 (modified 2021) |
|||||||||||||||||||||||||||