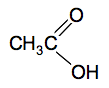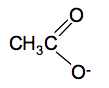|
Chemguide: Core Chemistry 14 - 16 Carboxylic acids This page introduces carboxylic acids as weak organic acids I am assuming that you have already read the pages about organic formulae and organic names. What are carboxylic acids? Names Carboxylic acids are members of a homologous series (family) of molecules which have an COOH group attached to a hydrocarbon chain. The first four carboxylic acids are:
Look carefully at how the names work. The part of the name which counts the number of carbon atoms includes the one in the COOH group. So ethanoic acid has 2 carbons ("eth"), no carbon-carbon double bonds ("an") and a COOH group ("oic acid"). It doesn't matter that you have already counted that carbon in the first part of the name. The old (and still used) common name of ethanoic acid is acetic acid and this is responsible for the acidity and smell of vinegar. Structures The complicated bit is the COOH group. The oxygens are written separately because they aren't bonded in the same way.
If you are asked for the structural formula in an exam, that is how you should write the COOH group. If you were asked for a fully displayed formula, then you would draw all the individual C-H, C-C and O-H bonds as well. Physical properties of carboxylic acids The pure acids are colourless liquids with boiling points above that of water. The only one you are likely to come across is ethanoic acid which smells very powerfully of vinegar. Butanoic acid (old name: butyric acid) is a revoltingly smelly compound - smelling like rancid butter, or vomit. You are unlikely to come across that in the lab! Carboxylic acid as weak acids Acids ionise in solution to form hydrogen ions. A strong acid like hydrochloric acid exists almost entirely as hydrogen ions and chloride ions in solution. A weak acid like ethanoic acid and the other carboxylic acids only ionise to a small extent in solution in water - typically only around 1% of the acid exists as ions at any one time. When you add concentrated ethanoic acid to water, this equilibrium is set up. CH3COOH(aq) The CH3COO- ions are called ethanoate ions and have this structure:
So ethanoic acid solution (and solutions of the other carboxylic acids) contain hydrogen ions, but not as many as hydrochloric acid of the same concentration. This shows in their reactions. Testing with pH paper Ethanoic acid solution typically has a pH in the region 2 - 3 depending on the concentration. That's significantly higher than the pH of hydrochloric acid of the same sort of concentrations. Simple reactions Dilute ethanoic acid reacts in the same way as acids like hydrochloric acid - just slower. The video shows a simple comparison between the two acids with magnesium ribbon and with solid sodium carbonate. The video uses the symbol 1M for the concentrations of the two acids. If you haven't done any chemistry calculations yet, you won't know what that means. It doesn't matter - they are the same concentration. Reaction with magnesium ribbon Hydrogen gas is produced and the magnesium reacts with the acid to give a colourless solution of magnesium ethanoate. 2CH3COOH + Mg Why is the formula for magnesium ethanoate (CH3COO)2Mg? The ethanoate ion has the formula CH3COO Why is it written that way round with the magnesium on the end? Because that's the way we do it! By writing it that way round, we keep the structure of the compound more easily understandable. Suppose we wrote it the same way around as we write things like magnesium sulfate, but still tried to show how it was joined up. It would look like this: Mg(OOCCH3)2 At first glance that doesn't obviously have anything to do with ethanoic acid. Reaction with solid sodium carbonate Carbon dioxide is produced and the sodium carbonate reacts to give a colourless solution of sodium ethanoate. 2CH3COOH + Na2CO3 Reaction with sodium hydroxide solution You would mix two colourless solutions and get another colourless solution of sodium ethanoate. A simple neutralisation reaction occurs. CH3COOH + NaOH Summary on the acid reactions Apart from the slightly awkward formulae of their salts, carboxylic acids just do the same sort of things as other acids you have come across - just more slowly. Get the formulae sorted out! Everything else is easy. Formation of esters - esterification Esterification involves a reaction between carboxylic acids and alcohols. This is a bit of chemistry that students often find confusing to start with, and I have given this topic a page of its own so that I can introduce it gently. If you want to go on to it now, you will find it by following this link.
© Jim Clark 2021 |
|||||||||