|
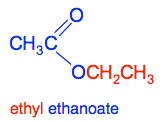
Chemguide: Core Chemistry 14 - 16 Esters This page introduces esters. Before you go any further, check your syllabus to find out how much (if anything) you need to know about esters. Several UK syllabuses don't mention them. I am assuming that you have already read the pages about organic formulae and organic names. You should also be familiar with alcohols and carboxylic acids. Don't go on with this page unless you are happy with all that. What are esters? Esters are derived from carboxylic acids. A carboxylic acid contains the COOH group, and in an ester the hydrogen in this group is replaced by a hydrocarbon group of some kind such as a methyl or ethyl group. Examples of esters - ethyl ethanoate The commonest ester you will come across is ethyl ethanoate. In this case, the hydrogen in the COOH group has been replaced by an ethyl group. The structure of ethyl ethanoate is:
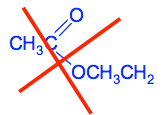
I have colour-coded the structure and name to show how they are related. If you were just writing this down in an equation you would write it as CH3COOCH2CH3 Names and structures of esters are a big stumbling block for people starting organic chemistry, because the structure is named the wrong way round from the way it is almost always drawn. If you have read the page about carboxylic acids, you will remember this is exactly what happens when you name sodium ethanoate, but write it CH3COONa, and the logic is the same. If you draw the structure reversed as CH3CH2OOCCH3, it no longer immediately looks as if it has anything to do with ethanoic acid. A word of warning! You can throw away a mark when you are writing the structural formula for an ester by writing the ethyl (or whatever) group the wrong way around. This is WRONG!
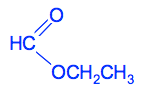
You have written the ethyl group the wrong way round! Does that matter? Yes it does. If you look at the number of bonds being formed by the carbon atoms in the ethyl group, the CH3 group is forming five: 3 to the hydrogens, 1 to the oxygen and 1 to the other carbon. The CH2 group is only forming three bonds: 2 to the hydrogens and 1 to the other carbon. Carbon has to form four bonds. Be careful! More examples of esters Naming a given ester Can you name this ester? CH3CH2COOCH3 Think about how this is made up. It has an acid group which will have come from CH3CH2COOH - propanoic acid. ("prop" because the acid has a total of 3 carbon atoms.) It also has a CH3 group - an alkyl group with only one carbon atom. So that's a methyl group. This is methyl propanoate. Working out a structure from the name Can you write the structural formula for propyl methanoate? Think about the acid bit first. Methanoate comes from methanoic acid. That's a carboxylic acid with only one carbon in the chain, including the COOH group. So methanoic acid is HCOOH. That means that the structure starts with HCOO. A propyl group has 3 carbon atoms ("prop" counts 3). So propyl methanoate is
Examples to work out yourself Name these esters: 1 CH3COOCH3 2 CH3CH2CH2COOCH2CH3 Write the structural formula for these esters, showing the bonding in the COO group: 3 ethyl methanoate 4 propyl propanoate Answers at the bottom of the page. Making esters Esters are made by reacting a concentrated carboxylic acid with an alcohol in the presence of a small amount of concentrated sulfuric acid as a catalyst. So to make ethyl ethanoate,you would mix
. . . and then heat the mixture. CH3COOH(l) + CH3CH2OH(l) Notice that the reaction is reversible. In the lab at this level, you would normally make up the mixture in a test tube and then heat it in a beaker of hot water. All the organic components of the mixture are flammable and ethyl ethanoate has a low boiling point. So you avoid having the tube in direct contact with a Bunsen flame. At this level, all you are likely to want to do is to smell the ester produced. Because it is only a part of a mixture which includes the strongly smelling ethanoic acid, the simplest way of getting the ester smell on its own is to pour the reaction mixture into a beaker of cold water after you have been heating for a few minutes. Everything else in the mixture dissolves in water, but the ester doesn't. Instead, it floats on the top as a very thin layer which you can smell easily. Ethyl ethanoate smells like a typical solvent. If you use nail varnish, for example, you will recognise the smell at once. You aren't likely to come across making other esters at this level. Smells and uses of esters The small ones smell like typical solvents, but as they get bigger, their smells become more fruit-like. Esters of various sorts are responsible for the smell and taste of all sorts of fruit. Ethyl ethanoate is important as a solvent. Bigger esters are important components of food flavouring and of perfumes. Answers to questions 1 methyl ethanoate 2 ethyl butanoate 3
4
If you didn't get any of these, work out what you did wrong and have another go later.
© Jim Clark 2021 |